Abstract:
Stainless steel is widely regarded for its corrosion resistance and is extensively used in construction, food processing, marine engineering, and medical equipment. However, real-world cases of stainless steel rusting—such as tea-colored rust spots on coastal handrails or pitting corrosion in 316L worktops—reveal that “stainless” does not mean “never rusts.” This article explains the underlying mechanisms, causes, and practical strategies to prevent stainless steel corrosion throughout its lifecycle.
1. The Protective Mechanism Behind Stainless Steel’s Corrosion Resistance
The key to stainless steel’s resistance to rust lies in its ultra-thin yet highly protective surface layer of chromium oxide (Cr₂O₃), known as the passive film. Although only 2–5 nanometers thick, it functions as an invisible armor.
Core Protection Mechanisms:
Physical Barrier: Isolates the steel from moisture and corrosive agents.
Chemical Stability: Cr₂O₃ is chemically stable and resistant to most acids and alkalis.
Self-Healing: If the passive film is damaged, chromium reacts with ambient oxygen to regenerate the protective layer.
However, this passive film is not invincible—under certain environmental or mechanical stresses, its integrity can fail, allowing corrosion to occur.
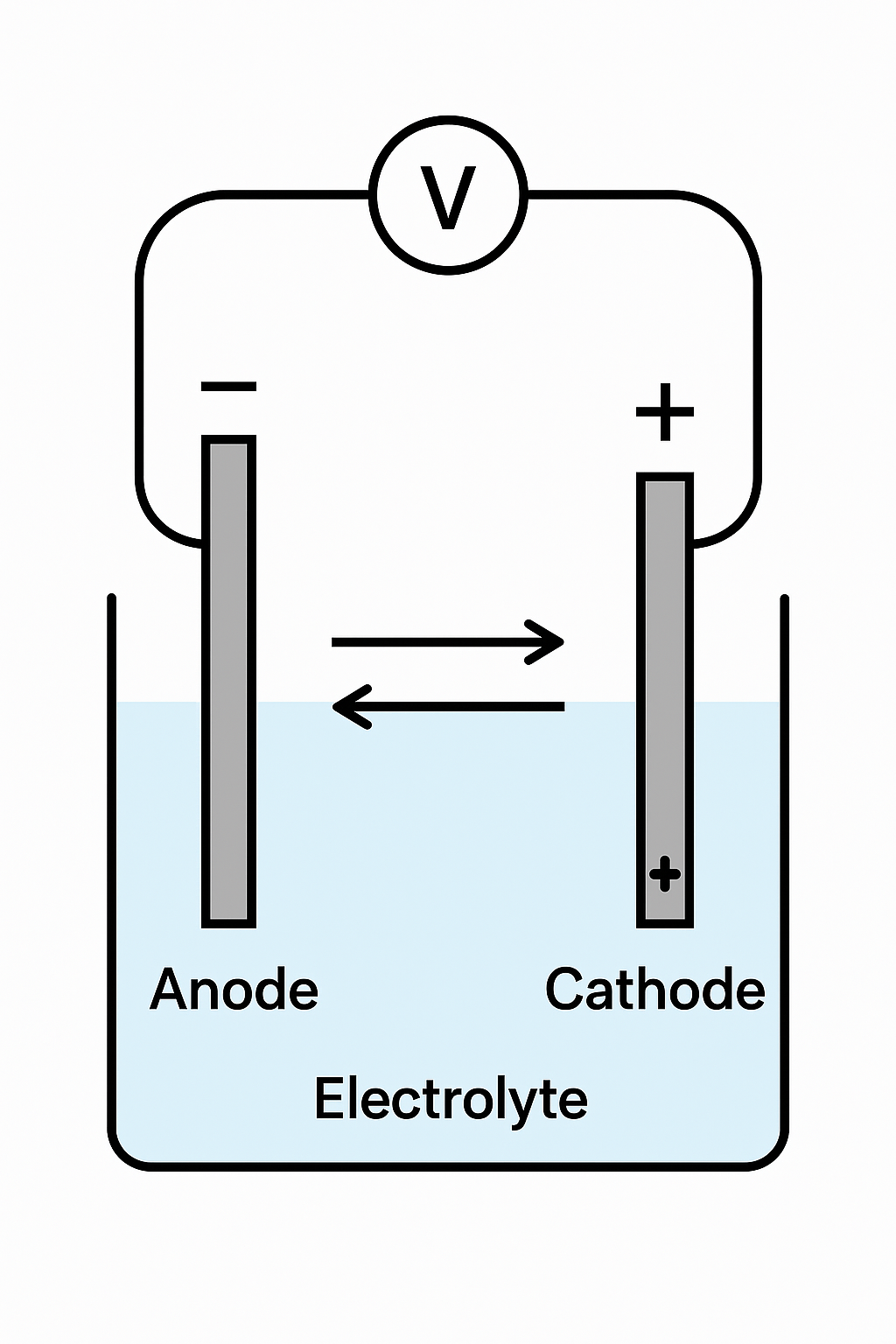
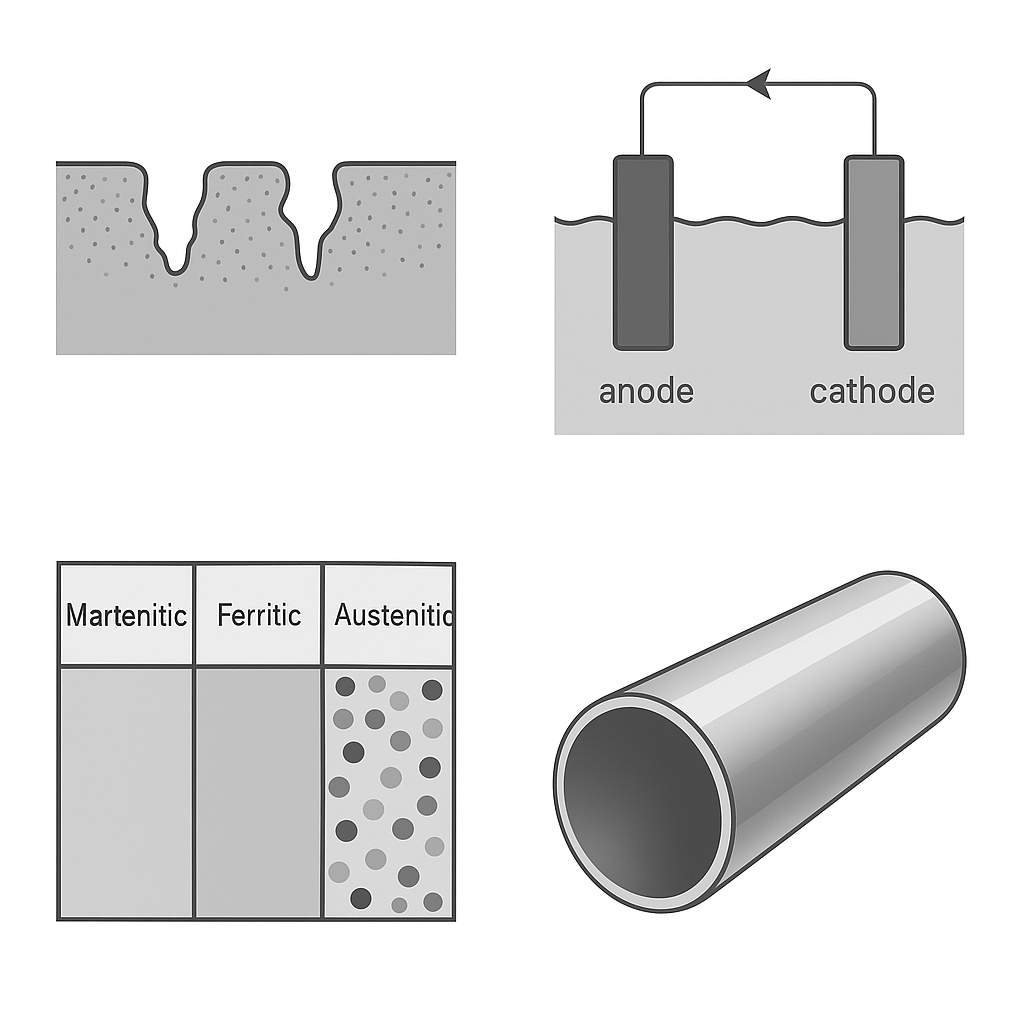
2. Common Causes of Stainless Steel Corrosion
2.1 Chloride Ion Attack (Cl⁻) – The Main Cause of Pitting
Chloride ions are small, highly polar, and can penetrate the passive film, forming soluble chlorides that lead to localized breakdown and pitting.
Typical Scenarios:
Coastal cities with seawater containing ~19,000 mg/L of Cl⁻.
Food factories using chlorine-based sanitizers.
Daily exposure to salt, water stains, or sweat.
Example: 304 stainless steel in coastal environments can corrode at a rate of 0.1 mm/year.
2.2 Mechanical Damage – Scratches as Corrosion Initiators
Surface damage such as scratches or abrasions exposes the steel substrate to oxygen and moisture, initiating localized corrosion. In low-oxygen zones like deep scratches, crevice corrosion or “occluded cell” corrosion can develop.
Example: A food-grade workbench cleaned with a steel brush developed pitting corrosion within six months, originating at scratch points.
2.3 Galvanic (Electrochemical) Corrosion
When stainless steel contacts dissimilar metals (e.g., carbon steel or copper) in a moist environment, galvanic cells may form, accelerating localized corrosion.
Scenarios Include:
Stainless steel pipes supported by carbon steel brackets.
Stainless equipment fastened with carbon steel bolts.
2.4 Sensitization – Chromium Depletion at High Temperatures
At 450–850°C, chromium reacts with carbon to form Cr₂₃C₆ (chromium carbide), depleting the chromium near grain boundaries. This weakens the passive film and causes intergranular corrosion.
Example: Stainless equipment that was welded but not properly heat-treated developed cracks along grain boundaries after 3 years in a chemical plant.
2.5 Aggressive Chemical Attack
While stainless steel resists most acids and bases, highly corrosive agents such as concentrated sulfuric acid, hydrofluoric acid, or hot NaOH can attack the passive film, causing rapid degradation.
2.6 Material & Manufacturing Defects
Chromium content below 10.5% significantly reduces corrosion resistance.
Excessive impurities (e.g., sulfur or phosphorus) promote pitting.
Poor welding, incomplete filler, or residual stress introduce long-term corrosion risks.
3. Comprehensive Anti-Corrosion Strategies
3.1 Smart Material Selection
| Environment | Recommended Stainless Type |
|---|---|
| Indoor, dry | 201, 430 (basic, cost-effective) |
| Urban atmosphere | 304 (18% Cr, 8% Ni; balanced performance) |
| Marine/high salinity | 316L (Mo-enhanced, strong chloride resistance) |
| Harsh chemicals, high temp | Duplex stainless steel (e.g., 2205) |
3.2 Surface Treatments to Reinforce Protection
Chemical Passivation: Enhances Cr₂O₃ film using nitric or citric acid treatments.
Electropolishing: Smooths surface, minimizing contaminant accumulation.
Coatings: PTFE and other protective coatings extend durability in harsh environments.
3.3 Proper Use & Maintenance Practices
Use neutral cleaners; avoid abrasive brushes or metal scouring pads.
Control humidity and inspect regularly for signs of rust.
Advanced tools like electrochemical impedance spectroscopy (EIS) can evaluate passive film integrity.
4. Advancements in Corrosion-Resistant Technologies
Emerging innovations are enhancing stainless steel’s performance in extreme environments:
Nanocrystalline Stainless Steel: Refined grain structure significantly improves corrosion resistance.
Self-Healing Coatings: Microencapsulated inhibitors release upon damage to rebuild protection.
Super Duplex Stainless Steel (e.g., SAF 2507): Superior to 316L in seawater and high-pressure environments.
Example: A Fork-11 tuning fork level switch uses a stainless steel enclosure to ensure long-term durability under corrosive industrial conditions.

5. Conclusion
The “stainless” nature of stainless steel is not absolute—it results from a dynamic balance between material properties and environmental factors. Ensuring long-term corrosion resistance requires:
Scientific material selection
Thoughtful engineering design
Precise manufacturing
Standardized operation and maintenance
By understanding corrosion mechanisms and implementing targeted protection strategies, stainless steel can remain both durable and aesthetically intact, upholding its status as the “silver medal” of modern industrial materials.
