What is the difference between standard condition and operating condition? How to calculate the gas state? How to convert the standard flow rate to the operating flow rate?

(I) The difference between standard conditions and working conditions
Working conditions: flow rate under actual working conditions, unit: m³/h
Standard conditions: flow rate under temperature of 20℃ and one atmosphere (101.325kPa), unit: Nm³/h
Note: The standard conditions usually refer to the conditions of temperature of 0℃ (273.15 Kelvin) and pressure of 101.325 kPa (1 standard atmosphere, 760 mmHg), which is different from the provisions of the standard conditions of industrial gases in my country.
The units in both conditions are the same, but the corresponding flow rates are different. In addition, the standard conditions referred to by different countries are also different.

(II) Calculation equation
According to the ideal gas state equation: pV=nRT.
This equation has 4 variables: p refers to the pressure of the ideal gas, V is the volume of the ideal gas, n represents the amount of gas substance, and T represents the thermodynamic temperature of the ideal gas; there is also a constant: R is the ideal gas constant.
PV/T=nR is a constant, so P1×V1/T1=P2×V2/T2
Assume that the volume flow rate under standard conditions is V0, temperature T0=273+20=293k, pressure P0=101.325Kpa=0.101325Mpa,
The volume flow rate under working conditions is V, temperature T (degrees Celsius), pressure P (gauge pressure, MPa), ignoring the change of compression factor, V*(P+0.101325)/(T+273)=V0*P0/T0
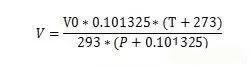
Note: Generally, natural gas is transported at medium and low pressures and enters households at low pressure. It is all under pressure and belongs to the working condition. The measurement of natural gas is measured according to the standard state (strictly speaking, it is the quasi-standard state, which we call the normal state).
The general trade measurement is measured by volume at 20℃ and 1 atmospheric pressure (0.1013MPa), which is slightly larger than the volume under the standard state, which is beneficial to the seller (because it is originally multiplied by 273, and at 20℃ it is multiplied by 273+20, so it becomes larger).
The standard state in the international standard is 0℃ and 1 standard atmospheric pressure. For gases, different pressures will have a large difference in volume (gas is very easy to compress), and of course the volume flow rate will be very different. The flow rate under different working conditions under the same diameter will naturally be very different.
For example, the maximum flow rate of the same diameter steam pipeline is different at 10bar and 3.5bar. Whether to use the working condition or the standard condition in process calculation depends on the chart you check and the constants you use.
Calculations of both states are possible. For example, when defining compressor parameters, we often use the parameters under standard conditions to provide conditions to manufacturers. At the same time, we also provide parameters such as temperature and atmospheric pressure for calibration under working conditions.
The advantage of doing so is that we can use the same state to indicate the parameters, just like the performance curve of a pump is expressed in terms of clean water. No one would say what the performance curve of gasoline is or what the performance curve of crude oil is. Working conditions are used in many calculations, such as when calculating flow rate.

III. Standard state of gas
The conversion formula for standard volume of natural gas is different from that of ordinary gas and must comply with the standards issued by China National Petroleum Corporation.
Gas equation:
Qn=Zn/Zg*(Pg+Pa)/Pn*Tn/Tg*Qg
Where:
Qn——Volume flow rate under standard conditions (Nm³/h)
Zn——Compression coefficient under standard conditions
Zg——Compression coefficient under working conditions
Pg——Gas pressure (KPa)
Pa——Local atmospheric pressure (KPa)
Pg+Pa——Absolute pressure under working conditions
Pn——Standard atmospheric pressure (101.325KPa)
Tn——Absolute temperature under standard conditions (national standard for natural gas 20℃) (293.15K)
Tg——Absolute temperature of the medium (273.15+t)K
t——Celsius temperature of the measured medium (℃)
Qg——Uncorrected volume flow rate (m³/h)
Note: The parameter with n is the standard condition parameter, and the parameter with g is the working condition parameter.
(IV) Conversion example between standard condition and working condition

Usually, the flow we refer to is the flow under standard conditions for the convenience of unified units; while the actual flow recorded in the factory operation is basically the flow under working conditions.
1. Example of conversion from standard flow to working flow:
Question: The rated gas output of the air compressor is 2 cubic meters per minute, and the pipeline pressure is 8 kilograms. What is the actual working flow of the pipeline?
Answer: Roughly calculate, assuming that the compressed air temperature is 20 degrees.
Working flow = 2/(0.8+0.101325)*0.101325=0.2248 cubic meters per minute
Where: 0.101325 is the absolute pressure of the atmosphere; 0.8 is the pipeline pressure, the unit is MPa.
2. Example of conversion from working flow to standard flow:
Question: The oxygen pipeline pressure is 12 kilograms, the working flow is 10 cubic meters per hour, what is the standard flow?
Answer: Assume that the temperature is 20 degrees and does not participate in the calculation.
Standard flow rate = 10/0.101325*(1.2+0.101325) = 128.43 cubic meters per hour
Where: 0.101325 is the absolute pressure of the atmosphere; 1.2 is the pipeline pressure, in MPa.

(V) Flowmeter selection calculation example
Question: The actual working pressure of a gas supply pipeline is known to be (gauge pressure) 0.8MPa~1.2MPa, the medium temperature range is -5℃~40℃, and the gas supply volume is 3000~10000Nm³/h (standard flow rate). Without considering the composition of natural gas, it is required to determine the specifications and models of the flowmeter.
Answer: According to the gas state equation, first convert the standard flow rate into the working flow rate, and then select the appropriate caliber. The gas equation is as follows:
Qb=Q×PTb/PbT×Zb/Zg=QCF2
Where: C is the conversion coefficient; F is the gas compression factor
Calculation:
① When the medium pressure is the lowest and the temperature is the highest, it should have the maximum standard volume flow rate,
That is, Qb=Q×PTb/PbT×Zb/Zg=QCF2=1200.87m³/h
② When the medium pressure is the highest and the temperature is the lowest, it should have the minimum standard volume flow rate,
That is, Qmin=213.51m³/h
From the above calculation results, it is known that the flowmeter to be selected has an operating flow range of (214-1200)m³/h.
According to the calculated flow range, select a flowmeter that meets the operating requirements.
