1. Introduction
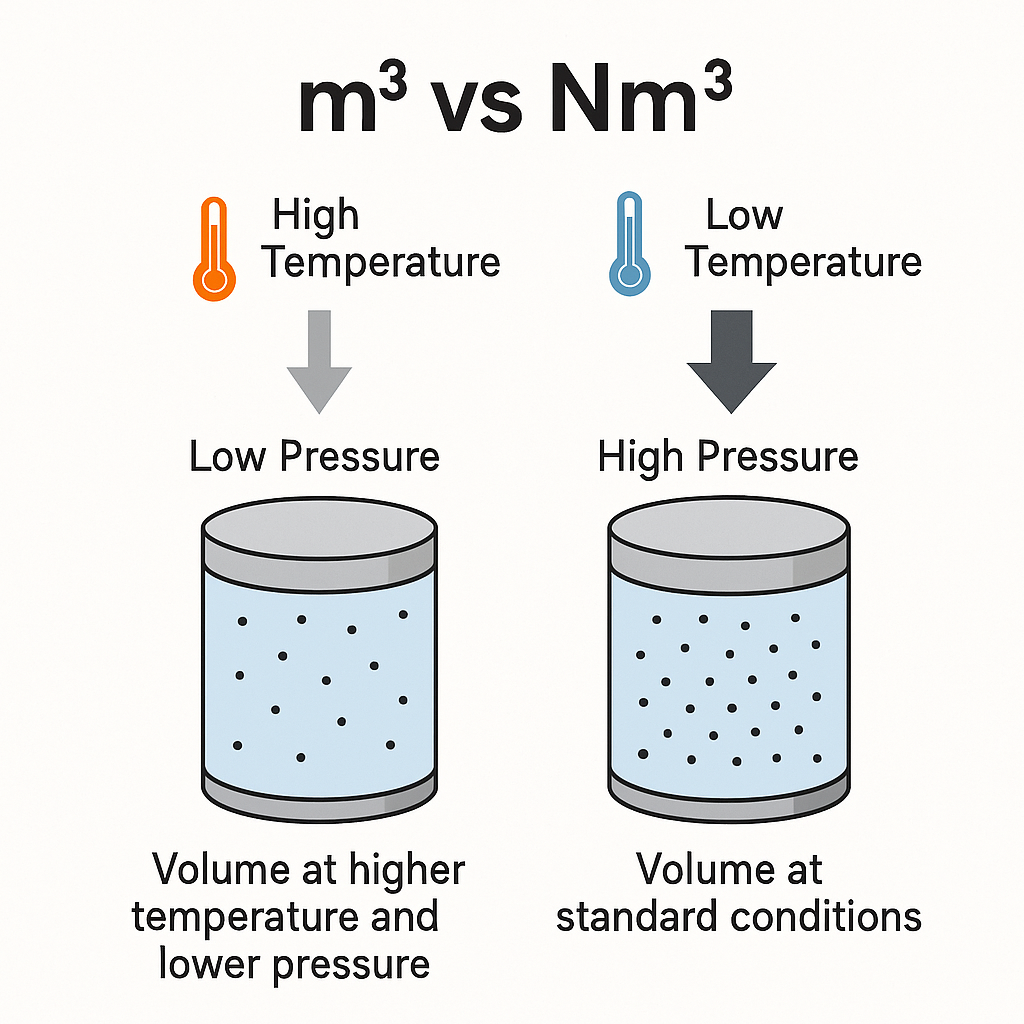
In industrial gas measurement, two units often appear in technical documents and trade contracts: m³ (cubic meter) and Nm³ (normal cubic meter).
While both express volume, their meanings differ significantly due to the influence of temperature and pressure. Understanding the distinction is essential for accurate measurement, energy calculations, and commercial transactions.
2. Definition of m³ and Nm³
| Unit | Meaning | Dependence on Temperature & Pressure | Typical Application |
|---|---|---|---|
| m³ | Volume of gas measured under the current operating conditions (actual temperature and pressure), also called “actual volume” or “operating volume.” | Directly affected by temperature and pressure at the time of measurement | Field flowmeter readings, process control |
| Nm³ | Volume of gas converted to standard conditions (normal temperature and pressure), also called “standard volume” or “normal volume.” | Corrected to a fixed reference condition | Trade settlement, energy billing, technical reporting |
3. What Is “Standard Condition”?
The reference temperature and pressure used to define 1 Nm³ are not globally unified. Common definitions include:
International Standard (ISO 13443):
Temperature = 0 °C (273.15 K)
Pressure = 101.325 kPa (absolute)Chinese Natural Gas Standard (GB/T 19206):
Temperature = 20 °C (293.15 K)
Pressure = 101.325 kPa (absolute)
Important: Always specify the reference conditions when reporting gas volumes in Nm³ to avoid misunderstandings.
4. Why the Difference Matters
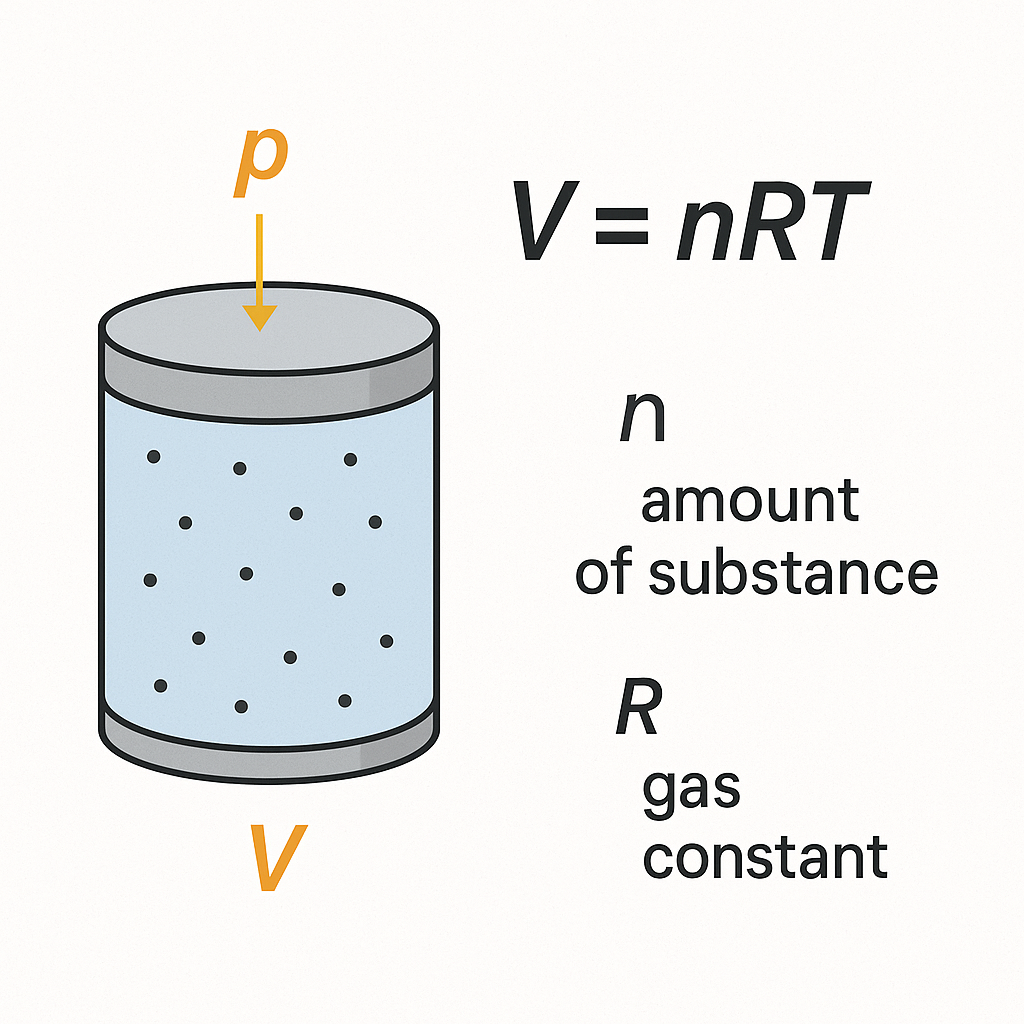
Gases are compressible and their volume changes significantly with temperature and pressure, according to the ideal gas law:
pV=nRT
At higher temperatures or lower pressures, a given mass of gas occupies a larger volume (m³ increases).
To make fair comparisons and accurate commercial settlements, volumes are converted to a common reference — the standard condition.
5. Conversion Formula
To convert from actual volume (VVV, m³) to standard volume (VNV_NVN, Nm³):
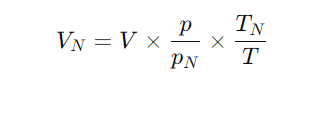
Where:
V = actual volume at operating conditions (m³)
p = absolute pressure at operating conditions (kPa or MPa)
pN = absolute pressure at standard condition
T = absolute temperature at operating conditions (K = °C + 273.15)
TN = absolute temperature at standard condition (K)
6. Example Calculation
Operating condition:
Temperature = 40 °C (313.15 K)
Pressure = 0.3 MPa (absolute)
Flow rate = 100 m³/h
Standard condition: 0 °C, 101.325 kPa
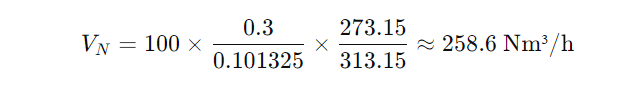
The standard volume is higher because the gas is less dense at the operating condition.
7. Practical Implications
Technical – For process control, m³ reflects the actual volume flowing through a system.
Commercial – For contracts, billing, and energy content calculations, Nm³ ensures fairness and consistency.
Regulatory – Some industries legally require reporting in Nm³ with the exact reference conditions specified.
8. Conclusion
m³ represents actual volume at current temperature and pressure.
Nm³ represents standardized volume, enabling consistent comparison and accurate trade settlement.
Always clarify the reference temperature and pressure when using Nm³ to avoid disputes or misinterpretation.
