Conductivity is a crucial property that describes a material’s ability to conduct an electric current, particularly in liquid solutions. It is an essential parameter in many scientific and industrial applications, including water quality testing, chemical analysis, and industrial wastewater management. Conductivity is typically measured in Siemens per meter (S/m), but depending on the context, microsiemens (µS/cm) or millisiemens (mS/cm) are commonly used. The principle behind conductivity measurement and its variations due to temperature changes can provide significant insights into the behavior of various solutions.

Principle of Conductivity Measurement
At its core, conductivity measurement relies on the fact that ions in a solution conduct electricity. These ions, both positively charged (cations) and negatively charged (anions), move towards electrodes of opposite charge when an electric field is applied. The more ions present in the solution, the higher its conductivity.
The most common method for measuring conductivity is the electrode method. This involves placing two electrodes into the solution and applying a voltage across them. The resulting current, driven by the movement of ions in the solution, is measured. The relationship between the current (I), voltage (V), and the physical properties of the electrodes (such as the distance between them and their surface area) allows for the calculation of conductivity using Ohm’s Law:
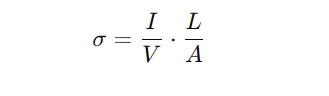
Where:
- σ is the conductivity,
- I is the current,
- V is the voltage,
- L is the distance between the electrodes,
- A is the cross-sectional area of the electrodes.
The conductivity value reflects the ease with which ions move in the solution. High ion concentration leads to high conductivity, whereas solutions with fewer ions, such as pure water, have low conductivity.
Temperature Compensation in Conductivity Measurements
Temperature has a significant effect on conductivity. As temperature increases, the mobility of ions in the solution increases, and this causes conductivity to rise. In general, the conductivity of most solutions increases by 2% to 3% per degree Celsius. To ensure accurate measurements across different temperatures, temperature compensation is used. This allows for the standardization of conductivity measurements to a reference temperature, typically 25°C.
The formula used for temperature compensation is:
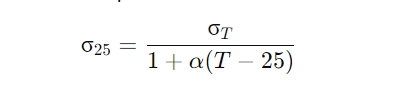
Where:
- σ25 is the conductivity at 25°C,
- σT is the measured conductivity at temperature T,
- α is the temperature coefficient (commonly 0.02 to 0.03 per °C),
- T is the current measurement temperature.
Most modern conductivity meters come with Automatic Temperature Compensation (ATC), where an integrated temperature sensor continuously monitors the solution temperature and adjusts the conductivity reading accordingly. This ensures that readings taken at varying temperatures can be compared consistently.
Typical Ranges of Conductivity
Conductivity varies significantly depending on the type of solution. The following are typical conductivity ranges for various solutions:
Ultra-Pure Water: 0.05 – 1 µS/cm. Ultra-pure water has very low ion content and, therefore, extremely low conductivity.
Drinking Water and Natural Water: 50 – 500 µS/cm. The mineral content in drinking water leads to higher conductivity than pure water.
Sea Water: 30 – 60 mS/cm. Due to the high concentration of dissolved salts, particularly sodium chloride, sea water exhibits very high conductivity.
Industrial Wastewater: 1 – 10 mS/cm or higher. Industrial effluents may contain high levels of dissolved salts and chemicals, resulting in significant conductivity.
Chemical Solutions: Vary widely, with dilute solutions of acids and bases having moderate conductivity (1 – 100 mS/cm) and concentrated solutions showing much higher values (100 – 800 mS/cm).
These ranges highlight the diversity in conductivity values, reflecting the different ionic compositions of these solutions. For instance, solutions with higher concentrations of electrolytes (such as sea water) exhibit much higher conductivity than those with fewer ions, like distilled water.
Applications of Conductivity Measurements
Conductivity measurements are widely used across various industries and scientific fields. Some of the common applications include:
Water Quality Monitoring: In environmental science, conductivity is used to assess the purity of water in rivers, lakes, and drinking water supplies. Higher conductivity can indicate contamination by salts or industrial pollutants.
Industrial Wastewater Management: Industries use conductivity to monitor and control the ion concentration in their wastewater before it is discharged, ensuring it meets environmental regulations.
Chemical and Pharmaceutical Processes: Conductivity helps in monitoring the concentration of ionic solutions in manufacturing processes, ensuring product quality and consistency.
Power Generation and Boiler Maintenance: Power plants use conductivity to monitor water quality in boilers and cooling systems to prevent scaling and corrosion caused by dissolved ions.
Conclusion
Conductivity is a fundamental parameter that offers valuable insights into the ionic composition and quality of solutions. Understanding the principles behind conductivity measurement, including how temperature influences readings, allows for accurate monitoring and analysis across a wide range of fields.
Whether in water quality testing, industrial applications, or chemical analysis, conductivity plays a vital role in ensuring that systems operate effectively and that environments are kept safe.
