When most people think about water purification, they picture filter cartridges, multi-stage filtration, activated carbon, or maybe UV lamps and softeners. In pharmaceutical manufacturing, however, this simplistic logic falls apart entirely.
Take any vial of injectable solution. Even if the label lists nothing but “salt” and “water,” the process behind producing that water involves a highly sophisticated and tightly controlled system: the Reverse Osmosis (RO) system.
This is not just a scaled-up household water purifier. It is a foundational component of the pharmaceutical quality assurance framework. A failure in this system could mean scrapping entire batches of medicine, shutting down production lines, failing audits, or even facing export bans.
Today, we will break down the core logic of pharmaceutical RO systems, why they are critical, how they operate, common pitfalls, and where the technology is heading.
In One Sentence: An RO system may look like water purification, but in reality, it is a war against uncertainty.
1. What is Reverse Osmosis (RO)?
RO is not filtration; it is molecular-level separation.
Unlike multi-layer filters, an RO membrane acts as a molecular sieve, forcing water through under high pressure and leaving nearly everything else behind.
Water molecules? Pass through.
Sodium, calcium, magnesium, chlorides? Blocked.
Bacteria and viruses? Blocked.
Organic pollutants, antibiotic residues, pyrogens? Largely removed.
This is not achieved with chemicals or heat, but through a purely physical, high-pressure process.
Core Components:
High-pressure pump
RO membrane module (an expensive consumable often costing thousands of dollars)
Think of the RO system as a “quality gateway” that only lets compliant water molecules through.
2. Why is RO Standard in Pharmaceutical Plants?
Because pharmaceutical manufacturers cannot afford to gamble on water quality.
Why not use distilled water?
Cost: Distillation delivers very high purity but is energy-intensive and requires complex equipment.
Control: Modern RO + EDI (Electrodeionization) + sterilization systems offer continuous operation with precise control.
Compliance: Pharmacopeias worldwide now accept RO systems as a validated method for producing Purified Water and even Water for Injection (WFI) in some jurisdictions.
Key Parameters Monitored in RO Systems:
Conductivity
Total Organic Carbon (TOC)
Pressure and flow rates
Water recovery rates
Summary: Water quality issues are not a source of revenue for drugmakers, but they are often the root cause of production failures.

3. Are Pharmaceutical RO Systems Complex?
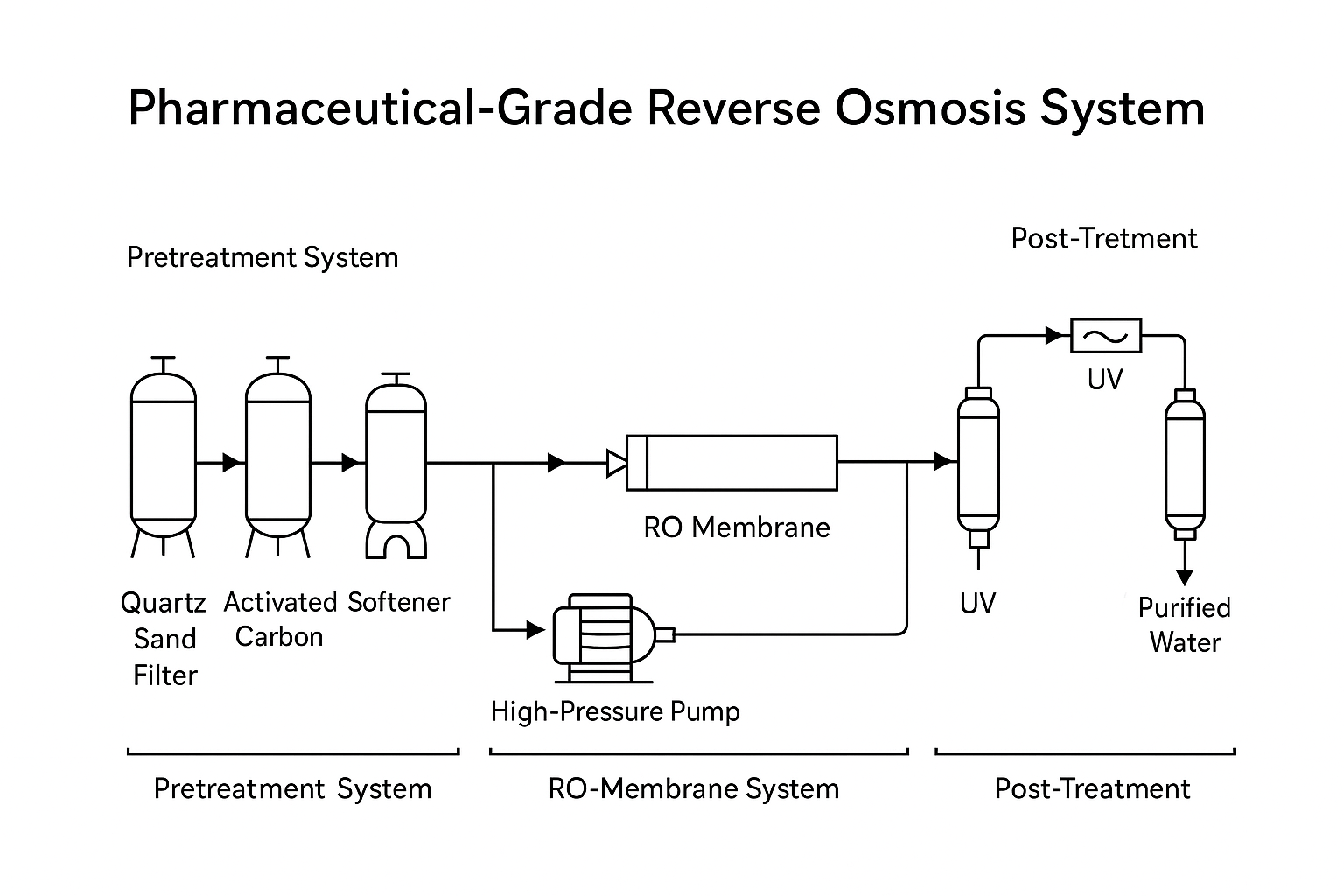
Far beyond “pump + membrane.” In pharmaceutical plants, RO is an integrated engineering solution with multiple subsystems:
✅ Pre-treatment Modules:
Quartz sand filters: Remove large particulates
Activated carbon filters: Adsorb organics and chlorine
Water softeners: Prevent scale formation on membranes
Micron filters: Protect the RO membrane from damage
✅ RO Membrane System:
Multi-stage design with precise control of pressure differentials (ΔP), recovery rates, and conductivity
Fully automated operation with real-time monitoring
✅ Post-treatment (Optional):
Second-pass RO for additional salt removal
EDI systems for ultra-pure water production
UV lamps for microbial control and TOC reduction
Terminal microfiltration (0.2μm) to prevent downstream contamination
Conclusion: The goal is not just achieving water purity, but building a stable, long-term, compliant water production line.
4. Misconceptions About Recovery Rate
Many non-specialists ask: “What is the recovery rate of your RO system?”
While higher recovery sounds better, it comes with risks:
High recovery = high concentration of contaminants = increased fouling risk
Fouling = higher maintenance costs + unstable water quality
Best Practice Design:
Recovery rates typically range from 50% to 75%
Some specialized systems achieve >80%, but these are rare and require careful engineering
Pharmaceutical facilities prioritize long-term stability over chasing maximum recovery numbers. A system that runs 3 years without membrane replacement is more valuable than one that achieves high recovery for a few weeks.
5. The Greatest Risk is Not Poor Water Quality
The real threat is lack of oversight and expertise:
Poor system design (missing pre-treatment, incorrect parameters)
Inadequate monitoring (pressure differential increases ignored, TOC alarms silenced)
No integration with plant-wide monitoring networks (isolated systems are harder to control)
Weak validation practices (incomplete IQ/OQ/PQ, poor record keeping)
At GMP audits, these issues often surface too late.
Key Takeaway: An RO system is not for convenience—it is an uncertainty control tool. The less attention it gets, the higher the risk of catastrophic failure.
6. The Future of Pharmaceutical RO Systems
Three key trends are shaping the next generation of systems:
Smart Operations and Predictive Maintenance
AI-driven monitoring to predict membrane fouling and schedule cleaning
Automatic parameter adjustments to prevent human error
Unified dashboards for TOC, conductivity, pressure, and flow data
Energy Efficiency Optimization
Energy recovery devices (ERDs) to reduce pump loads
Variable-frequency drives for adaptive pumping
Reducing energy consumption to <1 kWh per ton of water
Compliance-Centric Integration
Integration with MES/QMS for full traceability
Electronic records compliant with 21 CFR Part 11
Automatic documentation of all cleaning, maintenance, and deviations
In the future, RO systems will not be viewed as “equipment” but as critical compliance nodes within the pharmaceutical QA system.

Final Thoughts
Reverse Osmosis systems are among the most underestimated components in pharmaceutical plants. They run silently in the background—until they fail. And when they do, they can signal the collapse of an entire quality assurance framework.
To produce high-quality medicines, manufacturers rely on raw materials and formulations. But without water control, none of it matters. RO systems are the first line of defense in pharmaceutical quality.
In the coming years, companies that excel at managing their RO systems will increasingly resemble “data-driven factories”—fully controlled, auditable in real-time, and capable of anticipating risk.
In pharmaceutical manufacturing, mastering water means safeguarding your license to operate.
