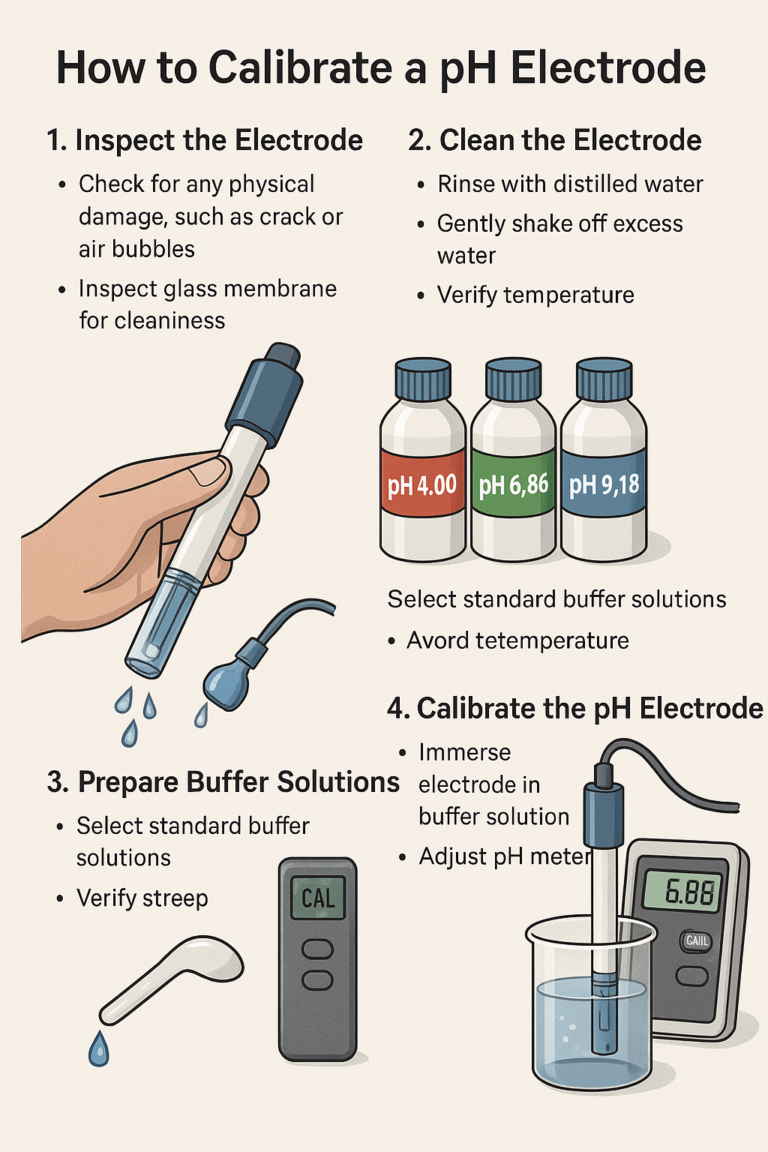
Calibrating a pH electrode is critical to ensure the accuracy and reliability of measurements taken by a pH meter. Below is a detailed guide to performing pH electrode calibration:
I. Preparation Before Calibration
1. Inspect the Electrode
Check carefully for any physical damage, such as cracks or air bubbles.
Ensure the internal reference electrolyte solution level is adequate (applicable to refillable electrodes).
Inspect the glass membrane to confirm it’s clean, free from contamination, or dried crystals.
2. Clean the Electrode
Rinse thoroughly with distilled or deionized water to remove residual contamination.
Gently shake off excess water without wiping, to prevent damage to the sensitive glass membrane.
3. Prepare Buffer Solutions
Select suitable standard buffer solutions, commonly used are pH 4.00, pH 6.86 (or 7.00), and pH 9.18 (or 10.01).
Always use fresh buffer solutions and avoid expired or contaminated solutions.
Calibration is usually done at 25°C. Ensure buffer solutions are close to this temperature. If the temperature differs, appropriate temperature compensation must be applied.
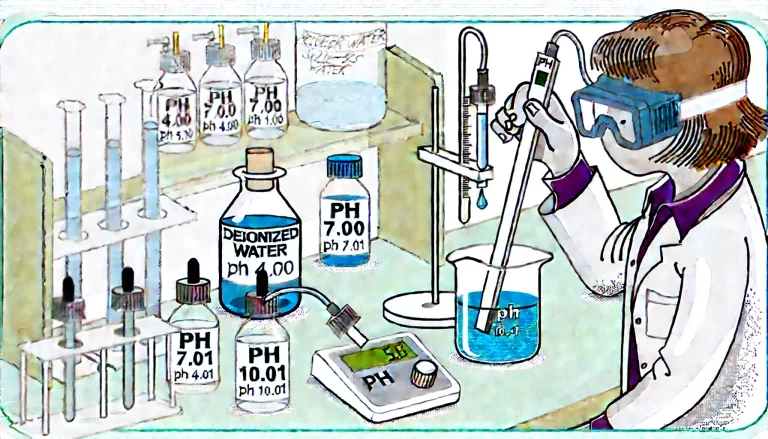
II. Calibration Procedures
Method A: Two-Point Calibration (Recommended)
Step 1: Neutral Buffer Calibration (pH 6.86 or 7.00)
Immerse the electrode in pH 6.86 or 7.00 buffer solution.
Stir gently to avoid air bubbles attaching to the electrode.
Wait until the reading stabilizes and adjust the pH meter to match the buffer solution’s specified pH value (automatic meters will auto-recognize).
Rinse the electrode with distilled water and gently shake dry.
Step 2: Acidic or Alkaline Buffer Calibration (pH 4.00 or 9.18/10.01)
Repeat the procedure with either pH 4.00 or pH 9.18 (or 10.01) buffer solution.
Verify the pH meter recognizes the buffer and completes calibration.
Step 3: Verify Calibration Accuracy
Re-measure the calibration buffer solution to confirm accurate reading.
If deviations occur, repeat the calibration process.
Method B: Three-Point Calibration (Higher Precision)
Following the two-point calibration, add an additional third calibration point (pH 4.00 or 10.01), particularly beneficial for precise measurements in strongly acidic or alkaline solutions.
III. Post-Calibration Maintenance
1. Electrode Storage
Long-term Storage: Store electrodes in designated electrode storage solutions or 3M KCl solution to prevent drying.
Short-term Storage: Store electrodes in pH 4.00 buffer or storage solution to maintain responsiveness.
2. Maintenance and Precautions
Always clean the electrode before and after each use with distilled water.
Regularly refill or replace internal electrolyte solutions in refillable electrodes.
If performance decreases, soak the electrode in specialized cleaning solutions.
IV. Troubleshooting Common Issues
| Issue | Possible Causes | Solutions |
|---|---|---|
| Drifting pH Reading | Electrode contamination or aging | Clean thoroughly or replace the electrode |
| Unstable readings | Electrode dryness or internal air bubbles | Soak the electrode in KCl solution and gently tap to remove bubbles |
| Inaccurate results post-calibration | Expired buffer solution or damaged electrode | Replace with fresh buffers and inspect electrode condition |
Regular calibration of the pH electrode following the above guidelines ensures consistent accuracy and reliability of your pH measurements.