Introduction
High-risk process units in the chemical industry refer to facilities that involve highly reactive chemical processes (e.g., nitration, sulfonation, halogenation, strong oxidation, diazotization, and hydrogenation) or operate under extreme conditions such as high temperature (≥300 ℃), high pressure (≥10 MPa), and cryogenic environments (≤−29 ℃).
In addition, high-risk storage units are facilities for storing toxic substances, liquefied hydrocarbons, liquid ammonia, flammable liquids with low flash points (≤−18 ℃), and pressurized liquefied gases.
These units present severe hazards including explosion, fire, toxic release, and catastrophic equipment failure, which can cause major industrial accidents.
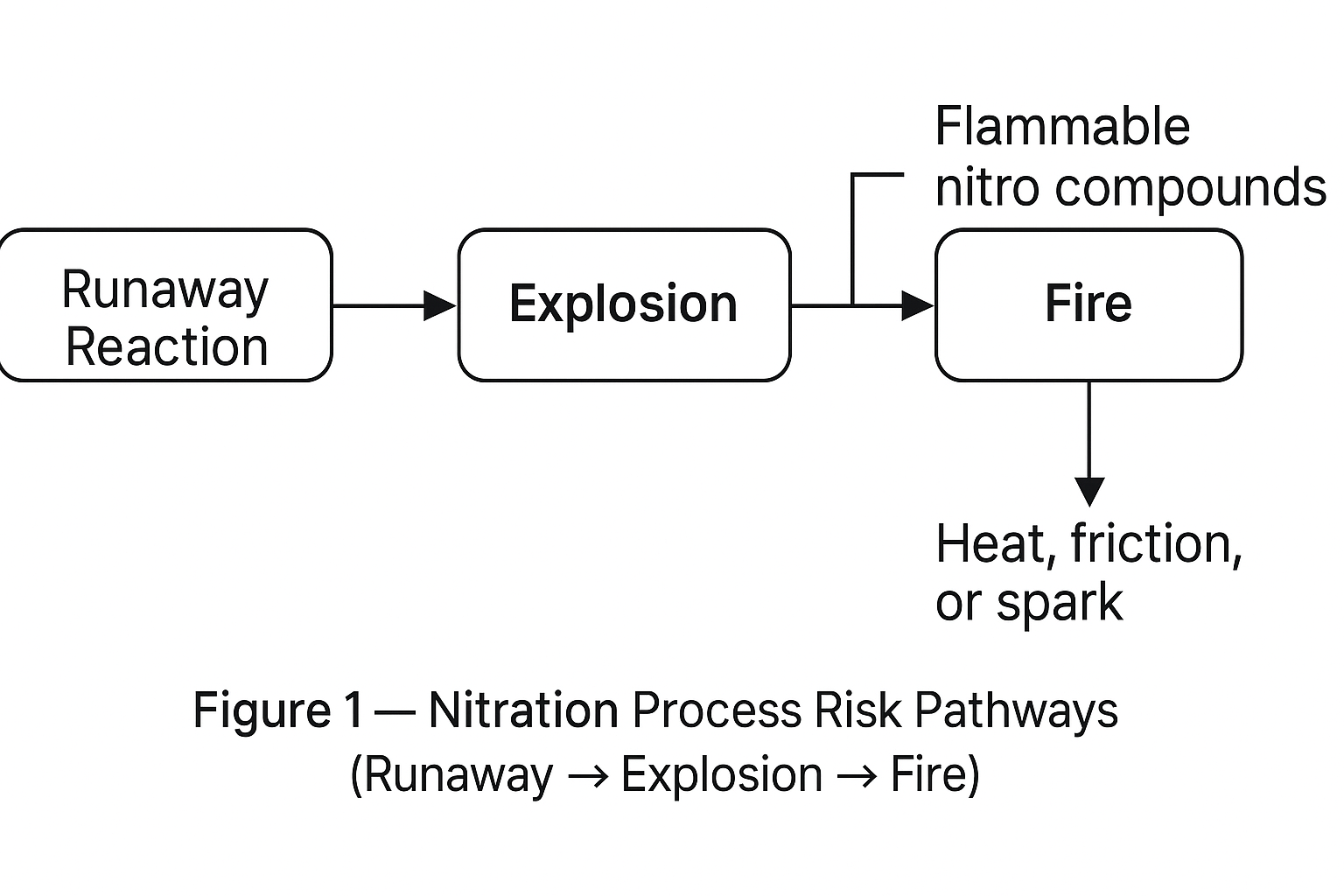
1. Hazards of High-Risk Process Units
1.1 Nitration Reactions
Process: Introduction of nitro groups into organic compounds (e.g., benzene nitration to nitrobenzene, glycerol nitration to nitroglycerin).
Main Hazards:
Explosion — Nitration is a highly exothermic reaction. Inadequate mixing, cooling failure, or rapid feed addition can cause runaway reactions.
Fire — Nitro compounds are typically flammable and toxic; heat, friction, or spark may lead to ignition.
Sudden Boil-over — Contact of mixed acid with water or unsaturated organics can cause violent boiling and ejection.
1.2 Sulfonation Reactions
Process: Introduction of sulfonic groups into hydrocarbons or polymers (e.g., alkane sulfonation, benzene sulfonation, chlorosulfonation of dyes).
Main Hazards:
Fire — Sulfonating agents (e.g., oleum, chlorosulfonic acid) are strong oxidizers; improper dosing or cooling failure can ignite raw materials.
Explosion — Strongly exothermic reaction; poor mixing or dosing imbalance can trigger thermal runaway.
Boil-over & Corrosion — SO₃ reacts violently with water, releasing heat and forming corrosive H₂SO₄.

1.3 Halogenation Reactions
Process: Substitution of hydrogen with halogen atoms (Cl, Br, F, I), e.g., phosphorus trichloride synthesis, brominated flame retardants.
Main Hazards:
Fire — Combustion risk when halogenating agents react with flammable organics.
Explosion — Strongly exothermic; lack of cooling may lead to pressure vessel rupture.
Toxic Exposure — Chlorine and bromine are highly toxic; leaks may cause mass poisoning.
1.4 Strong Oxidation Reactions
Examples: Ammonia oxidation to nitric acid, methanol oxidation to formaldehyde, propylene oxidation to acrylic acid.
Hazards:
Explosion — Intense exothermic behavior; peroxides formed as by-products may decompose violently.
Fire — Oxidizers can ignite under heat, impact, or contact with organics/acids.
1.5 Diazotization Reactions
Process: Conversion of aromatic amines to diazonium salts (precursors of azo dyes, initiators).
Hazards:
Explosion — Diazonium salts decompose rapidly under slight heat, light, or even room temperature.
Fire — Aromatic amines are combustible and may cause ignition during processing.
1.6 Hydrogenation Reactions
Process: Catalytic hydrogen addition for desulfurization, denitrification, or hydrocracking in petrochemicals.
Hazards:
Explosion — Hydrogen leakage under high temperature and pressure can cause catastrophic explosions.
Fire — Hydrocracking involves high hydrogen flow; leaks can form explosive mixtures.
Hydrogen Embrittlement — Steel equipment exposed to hydrogen at high temperature may lose strength due to carbide–hydrogen reactions.

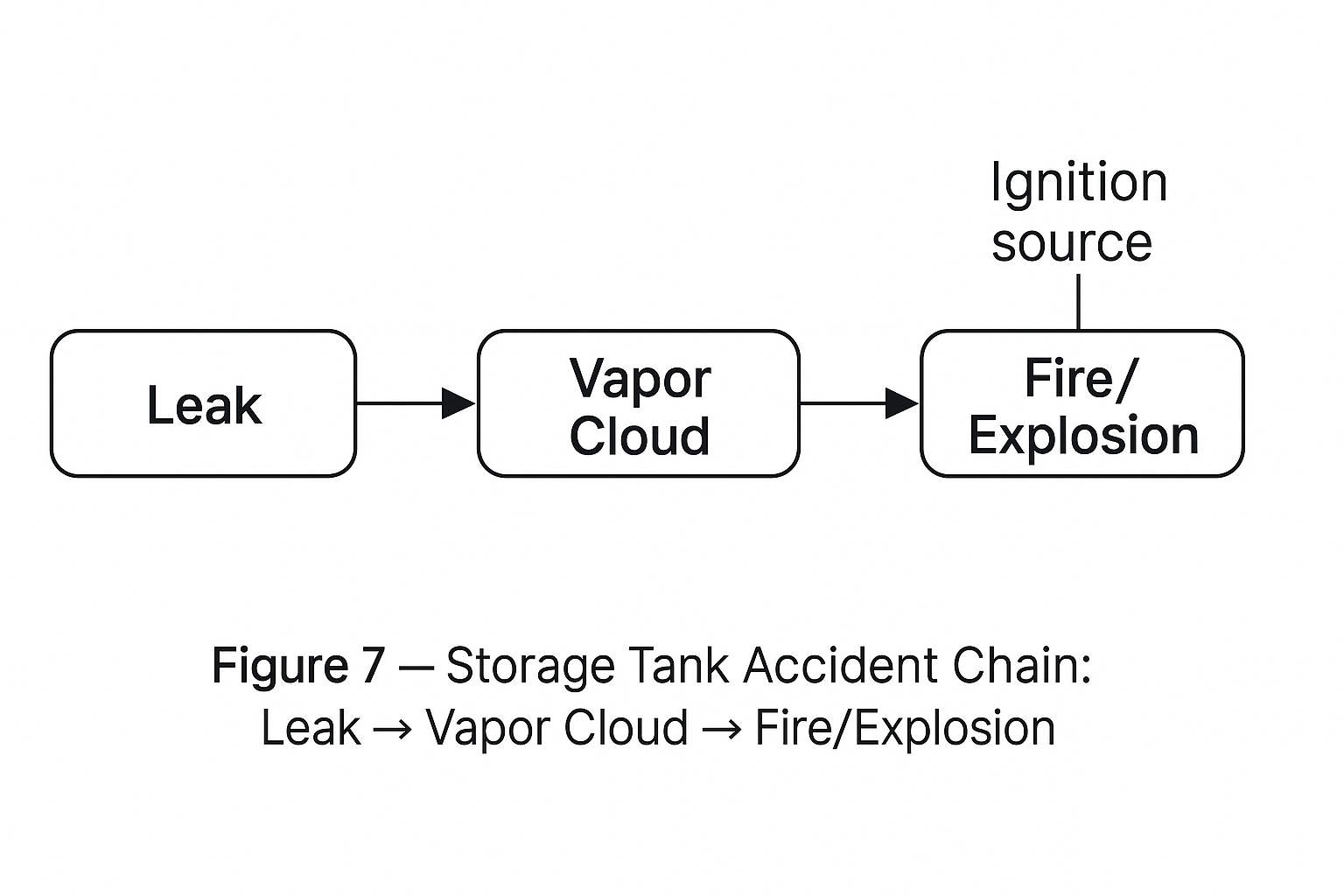
2. Hazards of High-Risk Storage Units
Leakage — Tank or pipeline failure may release toxic or flammable materials (e.g., chlorine tank leaks in Sichuan).
Toxic Cloud — Dense vapor clouds drift with wind, endangering plant workers and nearby communities.
Fire — Flammable liquids and reactive substances may ignite upon oxidation, friction, or water contact.
Explosion — Low-flash-point hydrocarbons (e.g., LPG tanks) may detonate from ignition or static discharge (e.g., Nanjing oil tank explosion).
3. Hazards of Manual Operation and Control
3.1 Five Key Hazard Factors
Material — Flammability, explosiveness, toxicity.
Inventory — Larger quantities → higher risk.
Temperature — High operating temperature increases ignition probability.
Pressure — High operating pressure increases equipment rupture risk.
Operation — Complex procedures increase risk of human error.
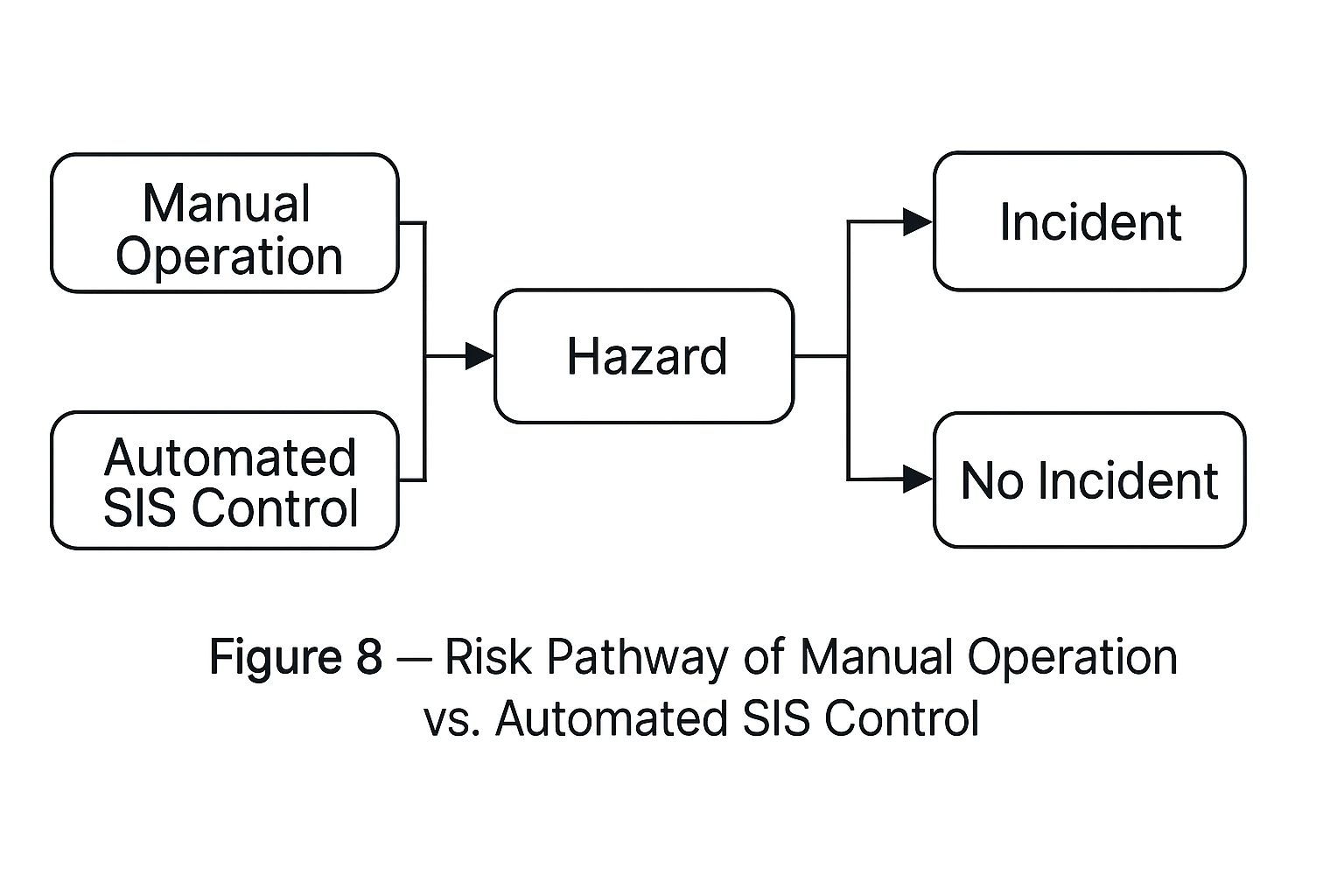
3.2 Risks of Manual Operation
High dependency on operator skill; misoperation of valves or dosing leads to overpressure or runaway.
Human error is a primary cause of incidents.
Difficult to maintain strict process parameters under manual control.
Operators exposed to toxic, hot, or electrically hazardous environments.
Poor equipment condition and safety management increase risk of mechanical injury, electric shock, falls, or poisoning.
Conclusion & Recommendations
High-risk process and storage units in chemical enterprises present multi-dimensional hazards including explosion, fire, toxic exposure, and equipment failure.
To mitigate risks, enterprises should:
Implement automation and Safety Instrumented Systems (SIS) to reduce reliance on manual control.
Strengthen emergency planning for toxic releases and fires.
Use corrosion-resistant and hydrogen-resistant materials for equipment design.
Conduct regular inspection, maintenance, and operator training.
Establish a safety culture and hazard identification system to continuously monitor risk levels.
