In chemical production, distillation, fractionation, and rectification are the most commonly used techniques for separating liquid mixtures. While they share the same fundamental principle—separation based on differences in volatility—they differ in their operational methods, equipment complexity, and application areas.
Understanding the connections and differences between these methods helps in selecting the most suitable separation technique, thus improving production efficiency and product quality.
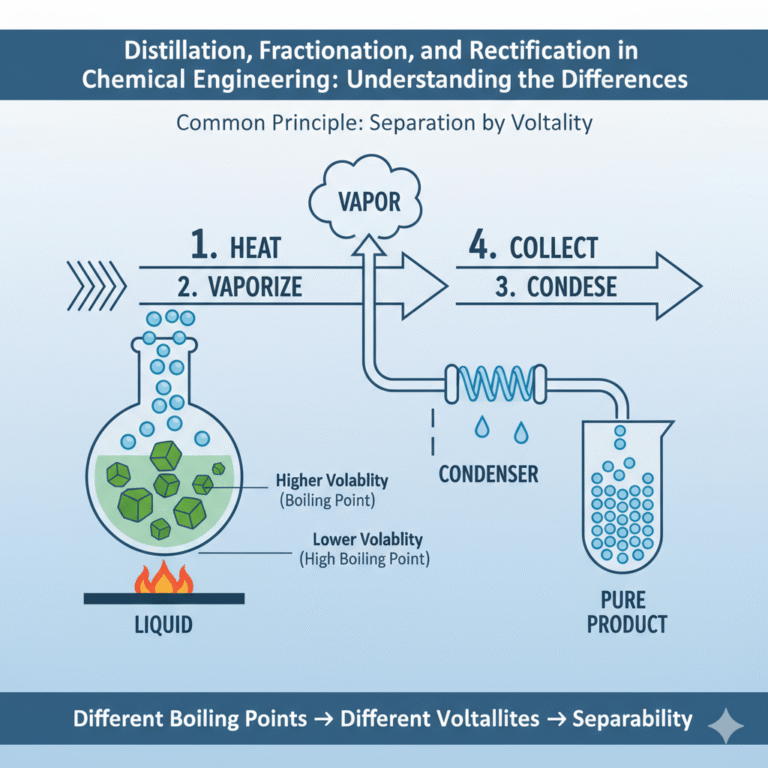
Common Principle
The basic principle of distillation, fractionation, and rectification is the same: separation occurs through the equilibrium process between gas and liquid phases.
When a liquid mixture is heated, components with higher volatility (lower boiling points) vaporize more easily, while those with lower volatility (higher boiling points) remain in the liquid phase. The vapor produced is condensed, separating the components. By repeating the vaporization and condensation processes, products of varying purity can be obtained.
In short, they all rely on the core mechanism of “different boiling points → different volatilities → separability.”
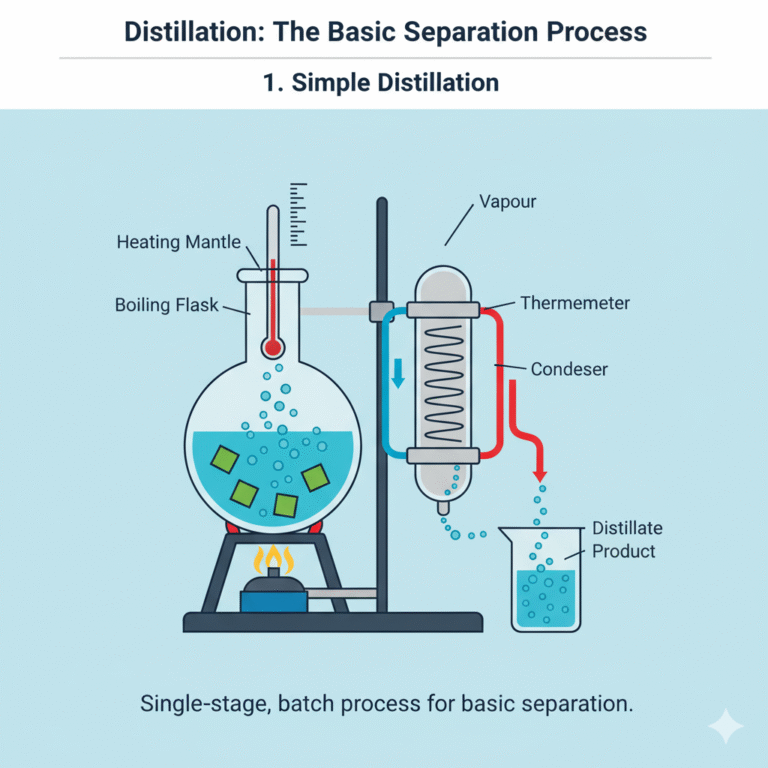
Distillation: The Basic Separation Process
Distillation is the most fundamental separation technique in chemical processes, and can be divided into simple distillation and equilibrium distillation (flash distillation).
1. Simple Distillation
Also known as differential distillation, this is a batch single-stage operation. The mixture is heated to boiling, and the vapor is drawn off and condensed into distillate. As distillation progresses, the volatile components in the boiling pot decrease, and the composition of the distillate changes with time. The operator typically selects the appropriate moment to collect the distillate, controlling its composition.
Characteristics & Applications:
Simple equipment, easy operation, low investment
Low separation efficiency, limited product purity
Suitable for small-scale operations or laboratory work, and initial separation of heat-sensitive materials (e.g., alcohol distillation in traditional brewing).
2. Equilibrium Distillation (Flash Distillation)
Equilibrium distillation is a continuous operation commonly used for industrial pre-separation. The mixture is heated and partially vaporized under reduced pressure, and the vapor-liquid phases are quickly separated and reach equilibrium. The vapor phase is rich in volatile components, while the liquid phase contains more of the non-volatile substances.
Characteristics & Applications:
Continuous operation, large throughput, simple equipment
Low separation accuracy, only preliminary separation
Commonly used in crude oil refining to separate initial products like gasoline, kerosene, and diesel.
Fractionation: Collecting Distillates in Segments
Fractionation can be seen as an extension of distillation. Like distillation, it relies on differences in volatility, but the focus is on collecting products in stages based on boiling point ranges.
During heating and condensation, different temperature ranges correspond to different components vaporizing and condensing. This allows products to be collected from multiple outlets according to their boiling point ranges.
Fractionation is especially important in petrochemical industries. For example, in a crude oil distillation tower, crude oil is separated into gasoline, kerosene, diesel, lubricating oil, and other products based on boiling point ranges. Each fraction has specific uses.
Fractionation places more emphasis on the classification of products and their suitability for particular applications, not just “whether separation is complete.”
Rectification: The Core Technology for High-Purity Separation
Rectification is an enhanced form of distillation that achieves efficient separation and purification through reflux. In a rectification column, liquid is heated at the bottom to produce vapor, which moves up through trays. The vapor contacts the descending reflux liquid, allowing for mass transfer and heat exchange. Each tray in the column acts as a small distillation stage, with the vapor becoming increasingly concentrated in volatile components, and the liquid accumulating more non-volatile substances. Ultimately, the top of the column produces high-purity light components, while the bottom yields high-purity heavy components.
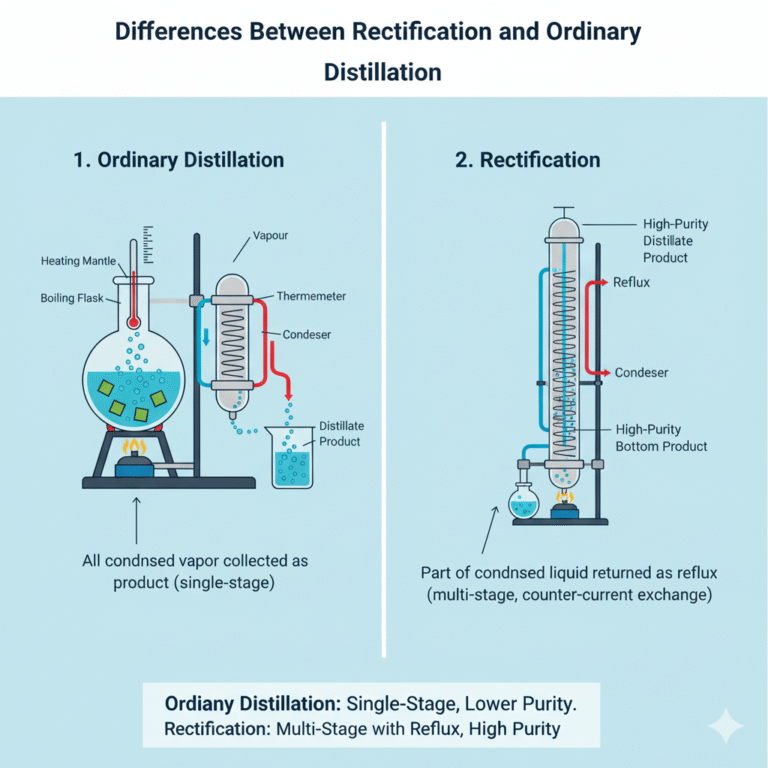
Differences Between Rectification and Ordinary Distillation
In ordinary distillation, the condensed vapor is directly collected as the product. In rectification, however, part of the condensed liquid is returned as reflux, allowing multiple exchanges of material and heat on each tray. The reflux process allows for multiple equilibria to occur, significantly improving separation efficiency. For example, while ordinary distillation may not achieve an ethanol purity higher than 90%, rectification can easily reach purities of 95% or higher.
Characteristics & Applications:
High separation efficiency, ideal for obtaining high-purity products
Continuous production, high automation
Complex equipment, higher investment and operational costs
Widely used in petrochemical industries, fine chemicals, pharmaceuticals, and food fermentation for product purification.
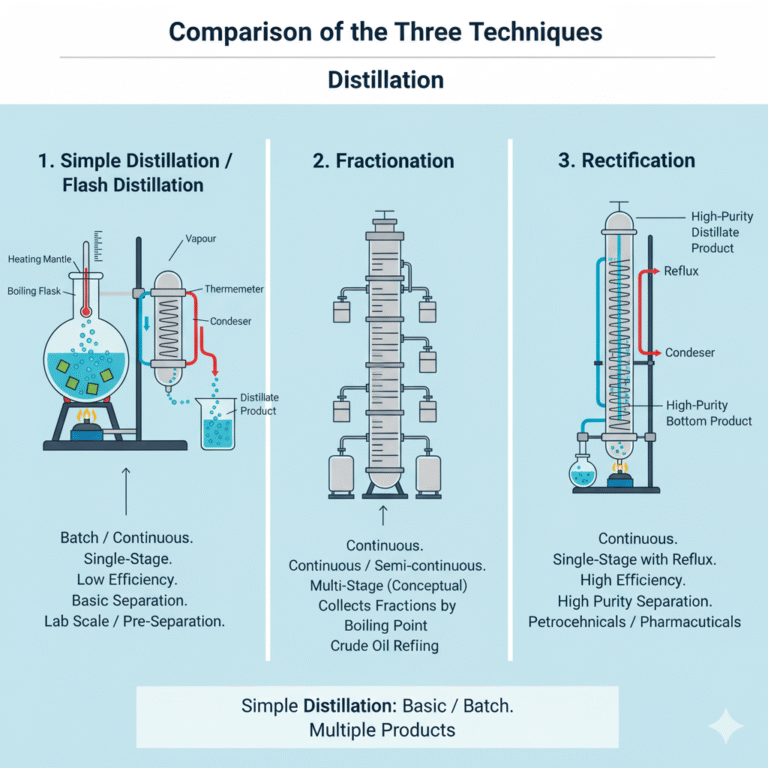
Comparison of the Three Techniques
| Method | Simple Distillation/Flash Distillation | Fractionation | Rectification |
|---|---|---|---|
| Operation Type | Batch (Simple) or Continuous (Flash) | Continuous or Semi-continuous | Continuous with Reflux |
| Separation Principle | Volatility difference | Volatility difference + Fractional Collection | Volatility difference + Multi-stage Mass Transfer |
| Equipment Complexity | Low | Medium | High |
| Separation Efficiency | Preliminary Separation | Moderate | High Purity Separation |
| Typical Applications | Alcohol Extraction, Initial Separation of Heat-Sensitive Materials | Crude Oil Fractionation, Chemical Fraction Production | High Purity Ethanol, Fine Chemical Purification |
Conclusion
These three separation techniques—distillation, fractionation, and rectification—have both a hierarchical and sequential relationship. Distillation is the most basic separation technique; fractionation builds on distillation by collecting distillates in segments; and rectification enhances distillation for high-purity product separation.
These techniques are widely used in chemical engineering, petrochemical industries, pharmaceuticals, and food processing. They form the core technologies of modern separation processes. Thanks to these methods, crude oil is refined into fuel, alcohol is purified into pharmaceutical-grade products, and chemical processes are made more efficient and precise.
