When selecting pressure transmitters, terms like “absolute pressure” and “gauge pressure” often cause confusion. A user once requested a pressure transmitter with a measurement range of 0~-90kPa absolute pressure, but was told that only a gauge pressure transmitter could be used. Why is that?
1. Absolute vs. Gauge Pressure: Understanding the Difference
A user wanted to purchase an absolute pressure transmitter with a range of 0~-90kPa. However, the manufacturer stated that this range would require a gauge pressure transmitter. Why?
The user’s request was for gauge pressure measurement, not absolute pressure. Absolute pressure and gauge pressure transmitters work differently:
Absolute Pressure Transmitter measures the pressure relative to absolute zero (vacuum). It is not affected by atmospheric pressure.
Gauge Pressure Transmitter measures pressure relative to atmospheric pressure.
In an absolute pressure transmitter, the pressure-sensing element is exposed to absolute vacuum on one side, whereas in a gauge pressure transmitter, the sensing element is exposed to atmospheric pressure on the other side.
If the user wanted to measure a -90kPa negative pressure, the absolute pressure could be calculated as follows:
For example, at sea level, the atmospheric pressure is around 101.325kPa, so:
This results in an absolute pressure range of 11.325 to 101.325 kPa.
However, the key point is that atmospheric pressure varies by location. In Lanzhou, for example, the atmospheric pressure is only 84.7 kPa, meaning that the maximum absolute vacuum is -84.7 kPa, not -90 kPa. This is why the user’s requested range is not feasible.
2. The Unit Conversion Issue: 1mmH2O = 9.087Pa vs. 9.789Pa
A common question arises about the conversion of 1mmH2O to Pa. According to unit conversion tables, 1mmH2O is approximately 9.087Pa. But why does the factory’s calibration report show 1mmH2O as 9.789Pa?
The answer lies in the density of water and the temperature at which the conversion is made. The standard conversion (1mmH2O = 9.807Pa) assumes a water density of 1.0g/cm³ at 4°C, with a gravitational acceleration of 980.665 cm/s². However, the factory performs the calibration at 20°C, where the density of water is 0.99823g/cm³. This slight difference in density results in a slightly higher conversion factor:
This is the correct conversion when water density is considered at 20°C.
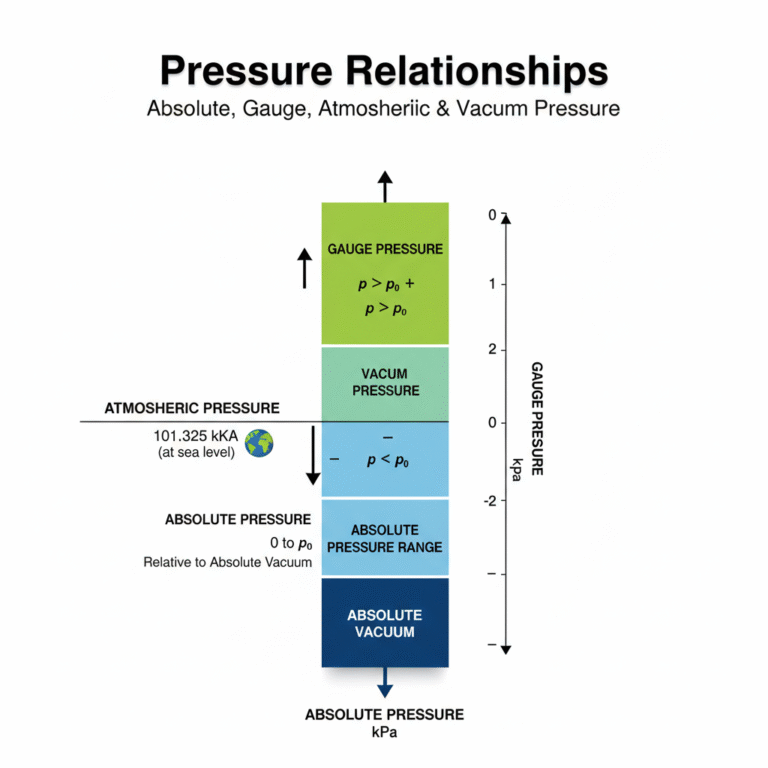
3. The Relationship Between Absolute Pressure, Atmospheric Pressure, Gauge Pressure, and Vacuum
Absolute Pressure is the pressure relative to absolute zero (vacuum), which is the baseline for all other pressure measurements.
Gauge Pressure is the pressure measured relative to atmospheric pressure (i.e., the difference between the measured pressure and the current atmospheric pressure).
Vacuum Pressure refers to negative gauge pressure, indicating that the pressure is below atmospheric pressure.
These pressures are interrelated. The diagram below shows the relationships between absolute pressure, atmospheric pressure, gauge pressure, and vacuum:
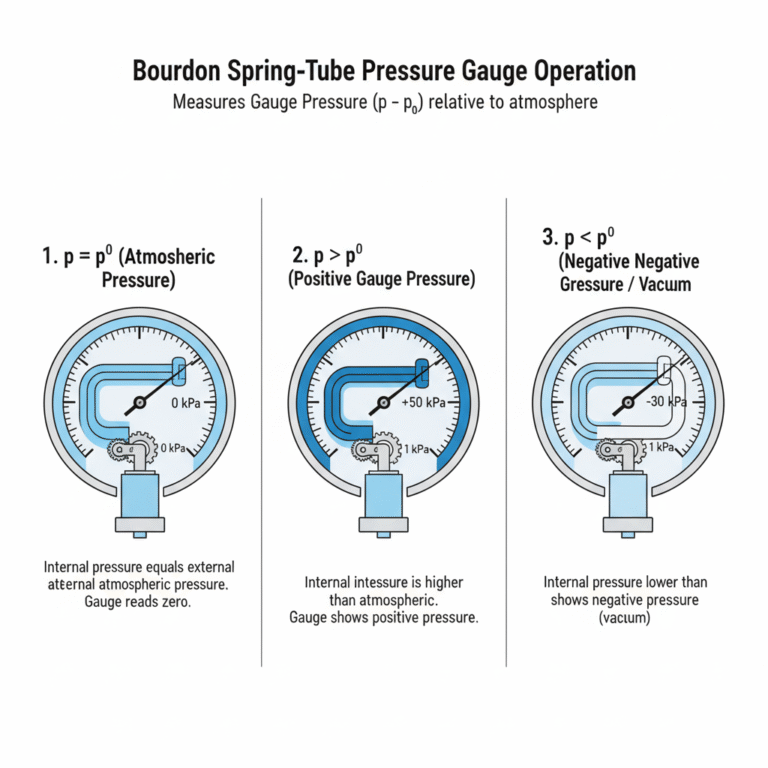
4. Why Spring-Tube Pressure Gauges Measure Gauge Pressure
Spring-tube pressure gauges measure gauge pressure because they compare the internal pressure (p) to the external atmospheric pressure (p₀). Here’s how the spring-tube pressure gauge works:
When the internal pressure (p) equals the atmospheric pressure (p₀), the gauge reads zero.
When the internal pressure (p) exceeds atmospheric pressure (p₀), the gauge shows a positive reading (p > p₀).
If the internal pressure is lower than atmospheric pressure (p < p₀), the gauge indicates a negative pressure (vacuum).
Thus, the spring-tube pressure gauge measures the difference between the measured pressure and the atmospheric pressure, which is gauge pressure.
