Anyone who works with materials can’t avoid one key term — creep.
Have you ever encountered this?
A plastic part slowly deforms over time?
A PTFE gasket that fit perfectly at installation ends up sagging after a few months?
Is this because the material is just too “soft”?
Or is there something deeper—perhaps molecular structure—that leads to this slow, chronic failure?
Today, we’ll take Polytetrafluoroethylene (PTFE) as an example and walk you through the entire mechanism—from its electronic structure down to macroscopic creep. We’ll answer these key questions:
What is creep, really?
Why is PTFE so “slippery”?
How do molecular interactions and electron clouds contribute to creep?
How can we scientifically prevent it?
1. What Is “Creep”?
Creep refers to the irreversible deformation of a material under constant load over time.
It’s not elastic deformation. It’s not brittle fracture.
It’s like dough slowly kneaded by time into a new shape.
Take a PTFE gasket, for instance. You tighten the bolts, walk away, and months later, you find the contour has sunken. That’s not a bad installation — it’s the molecular chains quietly slipping under long-term pressure and temperature.
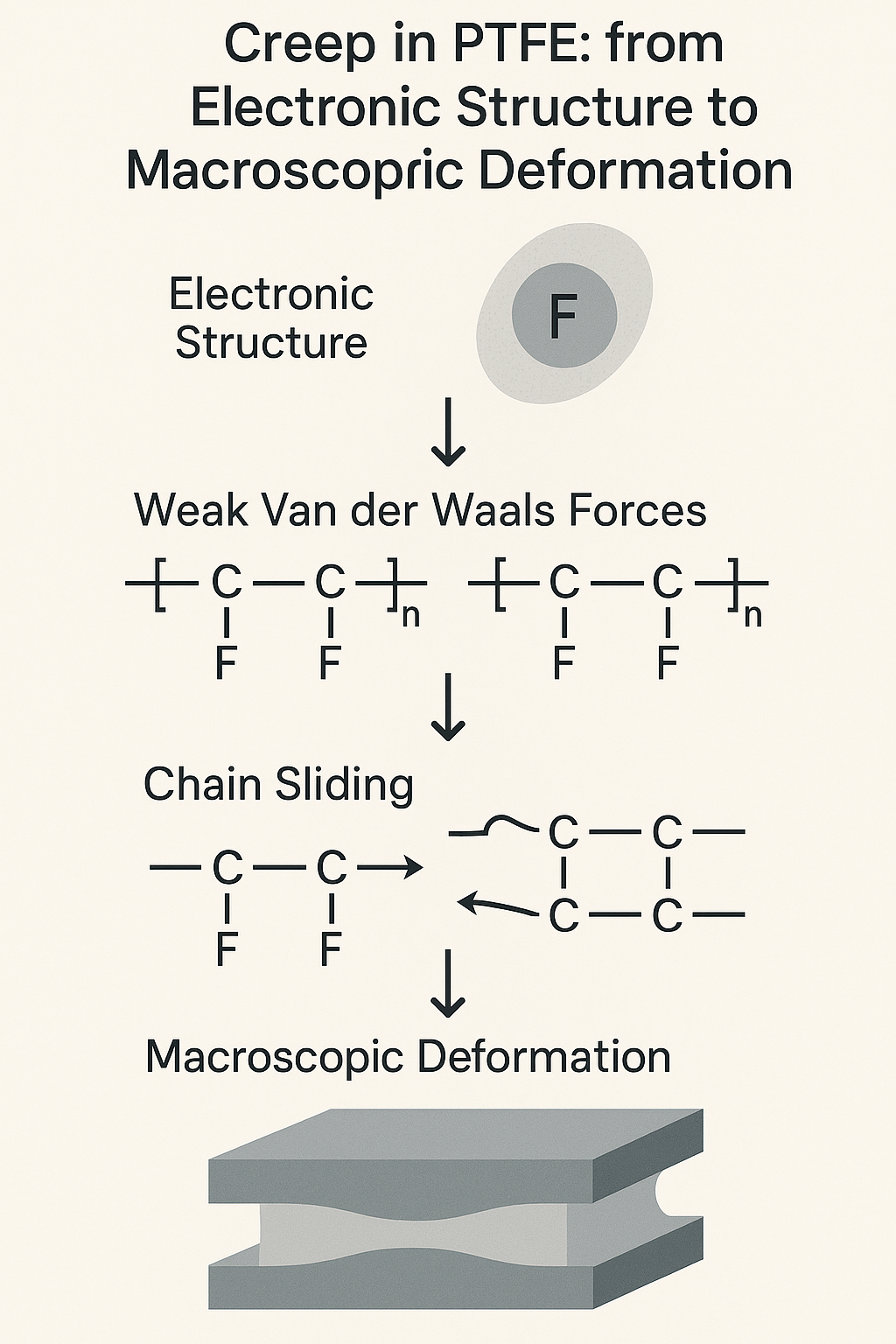
2. Why Is PTFE So “Slippery”?
Let’s look at the structure. PTFE has an extremely regular linear backbone:
Repeating unit:
–[–CF₂–CF₂–]–A chain of –C–C– links, each saturated with fluorine atoms.
This leads to three unusual properties:
Fully fluorinated – Fluorine is everywhere.
Highly non-polar – Almost no dipole moments.
Linear chain structure – Like a bundle of straight noodles.
As a result, PTFE chains barely stick to each other. Compared to other polymers:
No hydrogen bonding (unlike PA, PVA)
No dipole-dipole attraction (unlike PES)
No π-π stacking (unlike PI)
The only force left? Van der Waals forces—and even those are extremely weak.
Why so weak? Let’s dig deeper.
3. Why Is the Intermolecular Force in PTFE So Weak?
It all comes down to the nature of the fluorine atom:
Small atomic radius (~0.64 Å)
Extremely high electronegativity (3.98 – the highest of all elements)
Dense, symmetrical electron clouds
The dense electron cloud makes fluorine hard to polarize—meaning it’s difficult to form temporary dipoles. So even Van der Waals attractions are weak.
The result? PTFE chains behave like two polished marble slabs. There’s nothing to hold them together. They slide off each other with the slightest nudge.
This is why PTFE is commonly used for anti-corrosion coatings in instruments—it doesn’t stick to much of anything, chemically or physically.
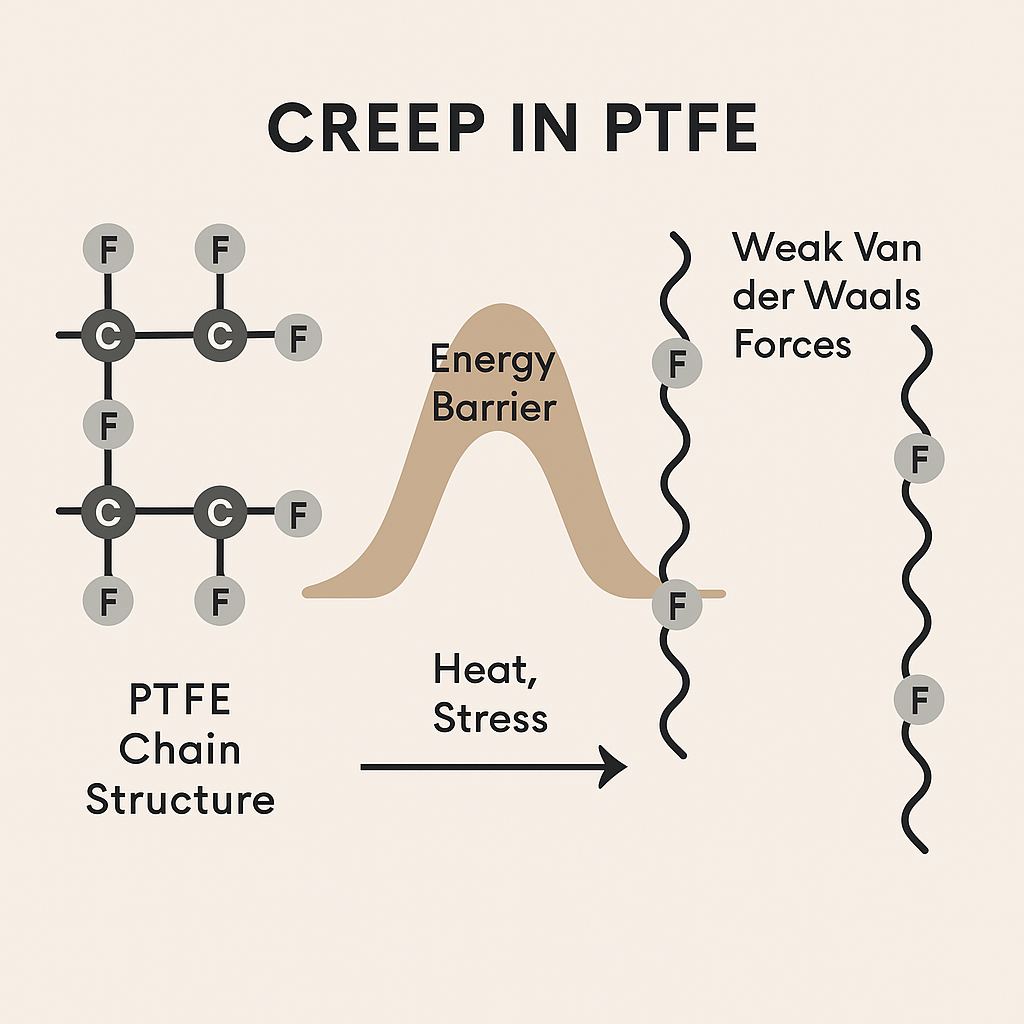
4. What Causes PTFE to Creep?
At the molecular level, creep = chain segment slippage.
Since PTFE’s intermolecular “braking” is so weak, even slight temperature or minimal stress can cause tiny shifts in the chains. Over time, these small steps lead to visible deformation.
This slipping happens not continuously, but in jumps:
Each segment is separated by a small energy barrier
Once thermal or mechanical energy exceeds the barrier, the segment jumps to the next position
In quantum mechanics, this is akin to phonon-assisted tunneling
To summarize in one sentence:
PTFE creep = thermally activated molecular “quantum jumps”, made easy by low inter-chain barriers.
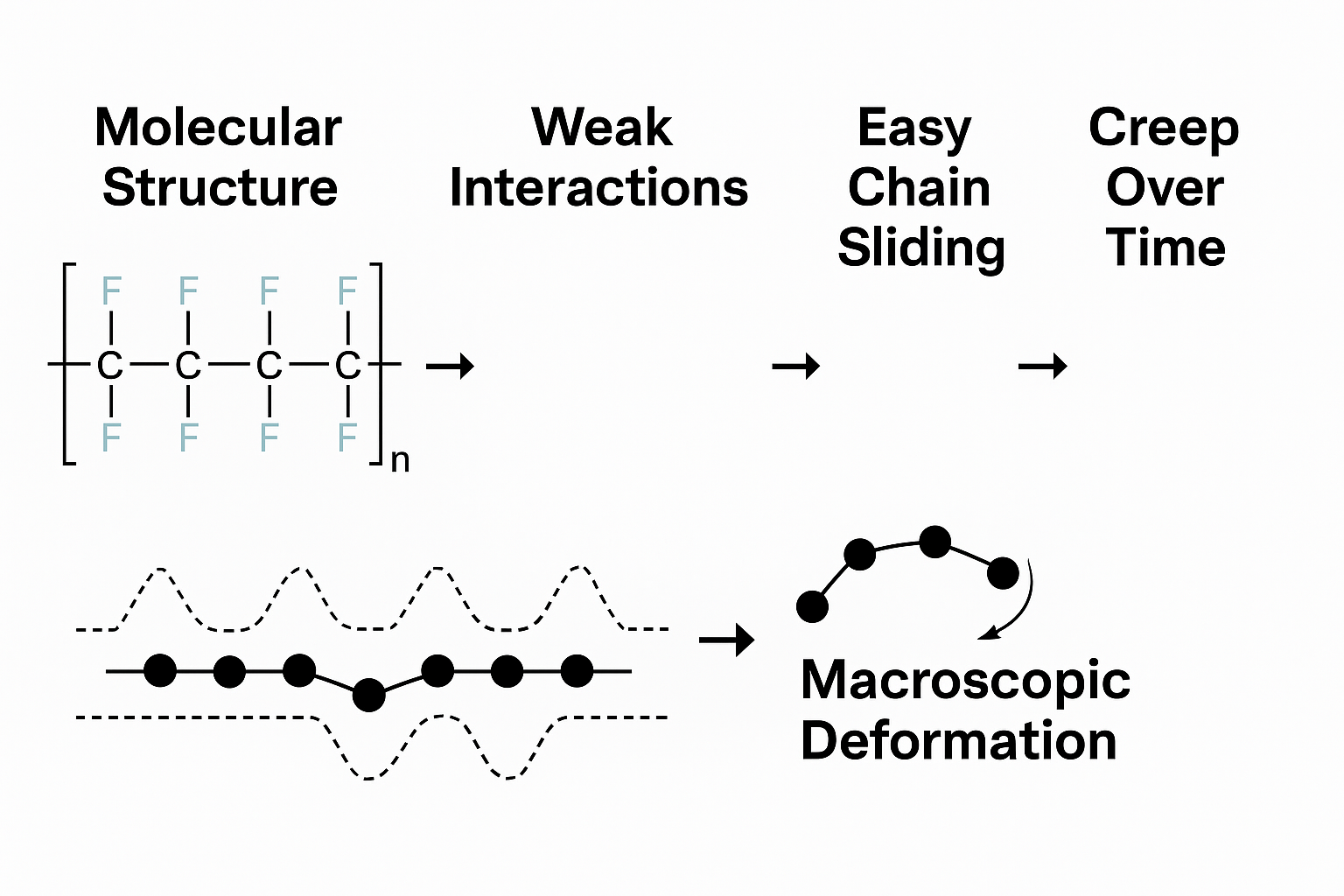
5. A Visual Map of the Logic
Molecular Structure → Weak Interactions → Easy Chain Sliding → Creep Over Time
6. How to Prevent PTFE Creep Scientifically
The core strategy is: Restrict chain mobility.
✅ 1. Add Reinforcements (Fillers or Crosslinkers)
Glass fiber, carbon fiber, or other fillers increase stiffness
They restrict molecular motion and enhance inter-chain interactions
✅ 2. Limit Operating Temperature & Stress
Higher temperatures accelerate creep
Although PTFE tolerates up to 260°C short-term, it’s best to keep continuous service temperature below 180°C
✅ 3. Use Composite Structural Design
Use PTFE as a lining or middle layer, enclosed by more rigid outer layers
The external materials apply physical constraints and reduce deformation risk
7. Summary
The incredible “slipperiness” of PTFE comes from its ultra-weak Van der Waals forces, stemming from its molecular and electronic structure.
This microscopic “freedom” eventually translates into macroscopic instability—what we observe as creep.
To use PTFE wisely, engineers must:
Understand its molecular behavior
Control the environmental conditions
Design around its structural weaknesses
With this, we can turn PTFE’s unique properties from a liability into an advantage.
