Steam plays an important role in industrial production and life. Its high efficiency, environmental protection and stability make it one of the indispensable energy sources in modern society. Usually, according to the physical state, we divide common steam into two categories: saturated steam and superheated steam.
Saturated Steam
Saturated Steam refers to the state in which, under a specific pressure, when a liquid (water) is heated to its saturation temperature, the water completely evaporates and becomes steam. At this time, the phase change between steam and liquid water is in dynamic equilibrium, that is, the evaporation rate of the liquid surface is equal to the rate at which the steam recondenses, and the density and temperature of the steam remain unchanged.
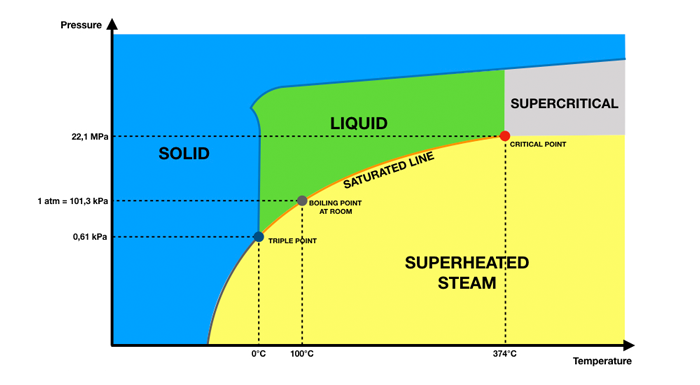
At 1 standard atmosphere (1atm), water vapor at a temperature of 100°C is saturated steam. There is a one-to-one correspondence between the temperature and pressure of saturated steam, and there is only one independent variable between the two.
The formation process of saturated steam is briefly introduced as follows:

When liquid (water) evaporates in a limited, enclosed space, water molecules pass through the liquid surface into the space above and become steam molecules. These steam molecules are in turbulent thermal motion, they collide with each other, and collide with the container wall and the liquid surface. When colliding with the liquid surface, some steam molecules are attracted by liquid molecules and return to the liquid to become liquid molecules.
As evaporation proceeds, the density of steam molecules in the space continues to increase, and the number of molecules returning to the liquid also gradually increases. When the number of molecules entering the space per unit time is equal to the number of molecules returning to the liquid, evaporation and condensation reach a dynamic equilibrium state, and the state at this time is the saturated state, and the corresponding steam is saturated steam.
The steam at the initial stage of equilibrium is wet saturated steam. After continuing to heat until the water in the saturated water is completely evaporated, dry saturated steam will be formed. The temperature of the steam does not increase in the process from wet saturation to dry saturation (that is, the temperature remains unchanged in the process from wet saturation to dry saturation).
Superheated Steam
Superheated Steam is steam obtained by further heating saturated steam to a temperature higher than the saturation temperature at that pressure. The temperature of superheated steam is higher than that of saturated steam at the same pressure. The temperature difference between the two is called superheat, which indicates the degree of superheat of superheated steam. The temperature and pressure of superheated steam are no longer fixed, but increase with the increase of heat.
Superheated steam has higher heat energy and heat capacity than saturated steam. It seems that superheated steam has more available energy than saturated steam. But in fact, if it is used as a working medium to convert heat energy into mechanical energy, its efficiency is indeed higher than that of saturated steam, but the efficiency of using superheated steam for heating is very low.
This is because superheated steam must be cooled to the saturation temperature before it can release the enthalpy of evaporation. The heat released from superheated steam cooling to the saturation temperature is very small compared to the enthalpy of evaporation.
If the steam superheat is relatively small, this small part of the heat is easier to release, but when the superheat is relatively large, the cooling time is relatively much longer.
In heat exchange equipment, the use of superheated steam will form dry walls in the heat exchange equipment, and the scale in the dry wall area will be faster, causing the tube wall to overheat and cause the tube to fail. Therefore, although the temperature of superheated steam at the same pressure is higher than that of saturated steam, its heating capacity is much lower than that of saturated steam.

What's the difference?
The difference between saturated steam and superheated steam lies mainly in their thermodynamic state. There are significant differences between the two in terms of temperature, pressure, heat and application.
1. Different temperatures
At the same pressure, the temperature of superheated steam is higher than that of saturated steam, that is, the temperature of superheated steam is higher than its saturation temperature. For example, at 1 standard atmospheric pressure, 100°C water vapor is saturated steam, and superheated steam needs to reach above 100°C.
2. Different pressure
At the same temperature, the pressure of superheated steam is higher than that of saturated steam. For example, the saturated steam pressure of 100°C water vapor is 1 standard atmosphere, while the superheated steam pressure is higher than 1 standard atmosphere.
3. Different calories
Superheated steam contains more heat than saturated steam, that is, the specific enthalpy of superheated steam is higher than that of saturated steam. For example, of equal amounts of saturated steam and superheated steam at the same temperature, the superheated steam has more heat.

4. Different stability
Since the temperature and pressure of saturated steam are fixed, saturated steam is more stable, while superheated steam is more prone to dangerous situations such as explosion.
5. Different ways of energy transfer
Superheated steam transfers heat by directly heating the object; saturated steam transfers heat through the phase change of matter, that is, the liquefaction and heat release process of water vapor.
6. Different application scenarios
Saturated steam is suitable for some relatively low temperature and low pressure application scenarios, such as heating, drying, cooking, etc.; while superheated steam has higher temperature and pressure, and is suitable for some application scenarios that require high temperature and high pressure, such as power plant boilers, steam turbines, etc.
