1. Introduction
In chemical processing environments, corrosion of instrumentation is often a direct result of inadequate material resistance to the working medium. Among the most aggressive chemical agents is sulfuric acid (H₂SO₄), which poses unique challenges to material selection due to its varying corrosive behavior across different concentrations and temperatures.
To ensure long-term reliability and accuracy of instruments exposed to sulfuric acid, careful analysis of corrosion mechanisms and precise material selection is essential.

2. Corrosive Behavior of Sulfuric Acid
Sulfuric acid displays nonlinear and dual-character corrosiveness:
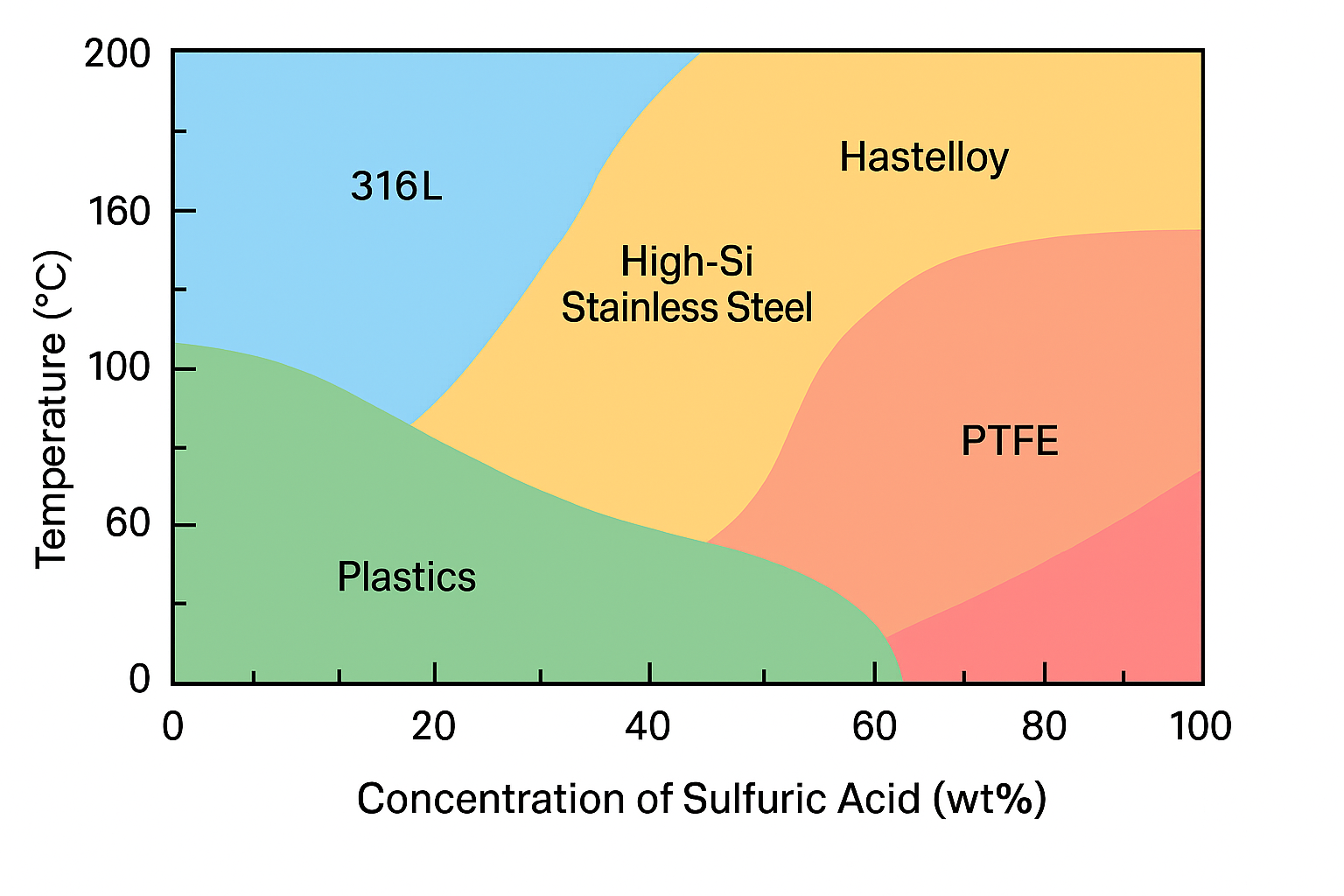
At low concentrations (<30%), it acts as a strong acid, aggressively attacking most metals.
At high concentrations (>90%), it shows strong oxidizing properties, allowing passivation of certain metals such as lead or tantalum due to the formation of a stable oxide film.
🔹 Influence of Temperature
Temperature significantly amplifies corrosion effects. Even materials that are normally resistant to sulfuric acid at ambient conditions may suffer accelerated degradation at elevated temperatures. For example:
Austenitic stainless steel such as 316L may resist diluted sulfuric acid at room temperature but fail rapidly when exposed to elevated temperatures.

3. Material Selection Guidelines
3.1 Recommended Materials for Different Sulfuric Acid Conditions
The following table summarizes the selection of stainless steel materials based on sulfuric acid concentration and operating temperature:
| Zone | Recommended Stainless Steel Grade | Application Notes |
|---|---|---|
| 1 | 0Cr18Ni12Mo2Ti, 1Cr18Ni12Mo2Ti | ≤40°C, air-free |
| 2 | 00Cr20Ni25Mo4.5Cu, 0Cr20Ni29Cu4Mo3 | ≤65°C |
| 3 | 0Cr18Ni18Mo2Cu2Ti, 00Cr18Ni14Mo2Cu2 | ≤50°C |
| 4 | 0Cr20Ni24Mo3Si3Cu2, 0Cr23Ni28Mo3Cu3Ti | ≤80°C |
| 5/6/7 | SS920 (High-Silicon Stainless Steel) | >90% H₂SO₄, ≤130°C |
| 8/9 | 1Cr18Ni9Ti, 0Cr20Ni29Cu4Mo3 | Used with carbon steel |
| 10 | — | Stainless steel not recommended |
Note: Selection should be validated against actual process conditions and impurities present.

4. Influence of Impurities
Sulfuric acid used in industrial processes may contain impurities such as chloride ions (Cl⁻), which can lead to:
Pitting corrosion
Intergranular attack
Stress corrosion cracking (SCC)
In such cases, materials with enhanced chloride resistance (e.g., Hastelloy, titanium alloys) may be preferred over traditional stainless steels.
5. Special Considerations
5.1 Non-Metallic Materials
For diluted sulfuric acid (<30%), certain plastics or elastomers (e.g., PTFE, PVDF, EPDM) may offer superior corrosion resistance and cost-effectiveness, particularly for lining, gaskets, and diaphragms in pressure or level transmitters.
5.2 Passivation Behavior
At higher concentrations (>90%), metals like lead (Pb) and tantalum (Ta) are often used because a passive film forms on their surface, inhibiting further corrosion. These materials, however, are less suitable for pressure-sensitive instrumentation due to mechanical limitations.

6. Recommendations for Instrumentation
| Instrument Type | Typical Contact Material | Recommended Material in H₂SO₄ Service |
|---|---|---|
| Pressure Transmitter | Diaphragm / Flange | 316L, Hastelloy C-276, PTFE lining |
| Level Transmitter | Wetted parts | SS920, PVDF, or glass-lined components |
| Flow Meter | Flow tube / Electrodes | PTFE lining + Hastelloy electrodes |
| Thermowell | Thermowell sheath | 0Cr20Ni25Mo4.5Cu, tantalum (if extreme) |
7. Conclusion
Choosing the right instrument material for sulfuric acid service is a balance between corrosion resistance, temperature tolerance, mechanical strength, and economic feasibility. Stainless steel with Mo, Cu, or Si enhancements can address many scenarios, but specific applications may still require high-alloy or non-metallic solutions.
Properly considering concentration, temperature, and impurity content—along with reference to corrosion data and material compatibility charts—is critical for successful, long-lasting instrumentation in sulfuric acid environments.
