Introduction
Sulfuric acid (H₂SO₄) is one of the most widely used chemicals in industrial processes, ranging from fertilizer production and petroleum refining to chemical synthesis and wastewater treatment. However, its strong corrosiveness poses a critical challenge for material selection and equipment design.
The corrosion behavior of stainless steels in sulfuric acid is primarily governed by acid concentration and temperature. Understanding these relationships enables engineers to make informed choices, avoid premature failures, and ensure safe long-term operation.
1. Corrosion Behavior of Stainless Steel in Sulfuric Acid
Sulfuric acid exhibits different corrosion characteristics depending on concentration and temperature. These can be classified into three zones: Passive (Safe Zone), Active (Dangerous Zone), and Transition Zone.
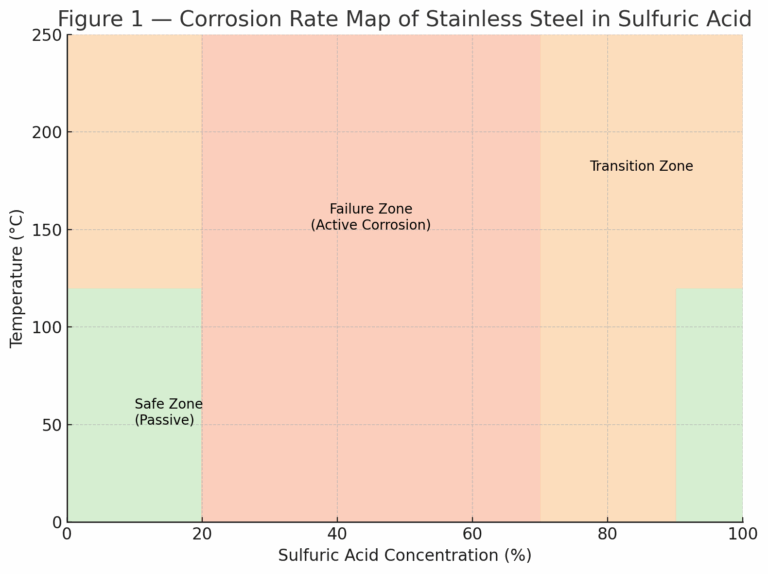
1.1 Passive (Safe Zone)
Dilute acid (<20%) at low temperature: Stainless steels form a stable passive film; corrosion rate <0.1 mpy (≈0.0025 mm/year). Grades such as 304, 316, 317, and 310 can be used safely.
Concentrated acid (>90%) at low temperature: Despite the aggressiveness of concentrated acid, a protective passive film remains stable. Stainless steels like 316 can operate effectively, making this range economically favorable for equipment.
1.2 Active (Dangerous Zone)
Medium concentration (20–70%): Corrosion rate exceeds 100 mpy (≈2.5 mm/year).
The passive film is severely damaged, and all common stainless steels fail rapidly.
This is often referred to as the “Failure Zone” for stainless steel and must be avoided in design.
1.3 Transition Zone
Between safe and dangerous regions, corrosion resistance is highly sensitive to temperature and concentration.
317 and 310 stainless steels show relatively better performance in certain conditions.
304 and 316 often fail quickly under the same conditions.
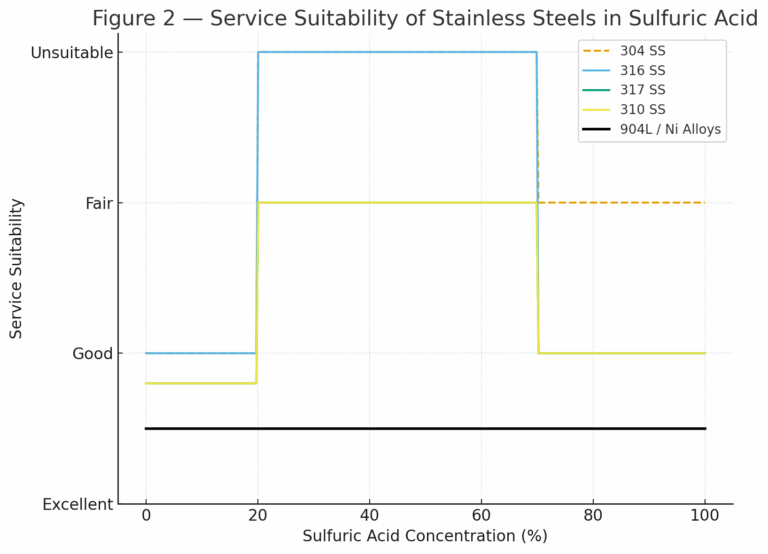
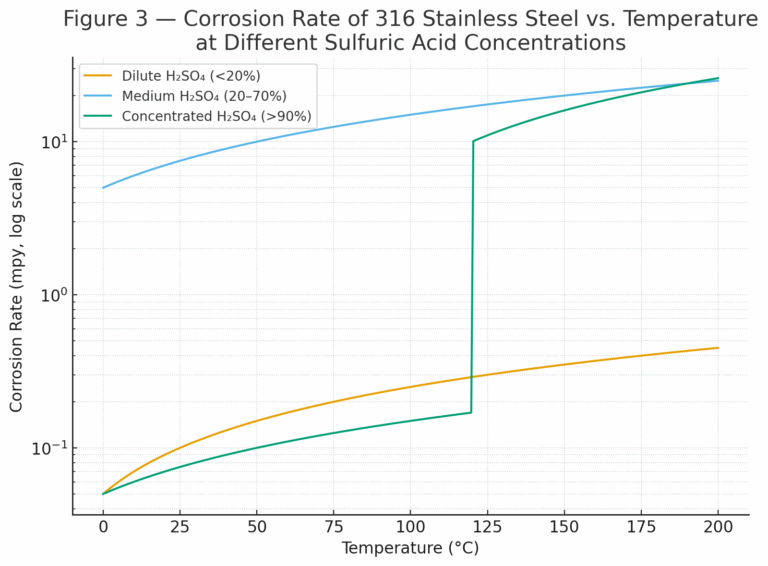
2. Service Conditions and Limitations of 316 Stainless Steel
316 stainless steel (UNS S31600/S31603) is commonly applied due to its good corrosion resistance in many acidic environments. However, its performance in sulfuric acid is strongly constrained by temperature and concentration:
Concentrated H₂SO₄ (>90%) at ≤120 °C:
Corrosion rate remains <0.1 mpy.
Safe for long-term service in tanks, piping, and equipment.
Temperature >120 °C or concentration <70%:
Rapid transition to the active corrosion region.
Severe material degradation may occur within weeks or months.
Alternative alloys must be considered.
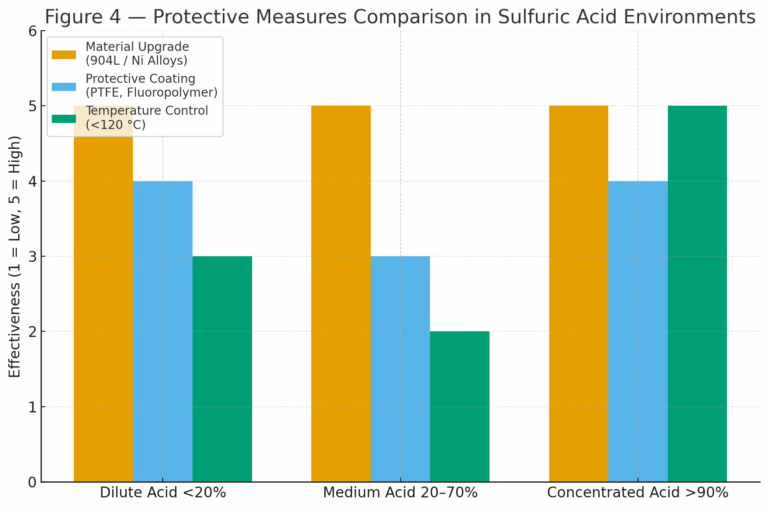
3. Engineering Strategies to Improve Corrosion Resistance
3.1 Use of Higher Alloy Materials
Super austenitic stainless steels (e.g., 904L, UNS N08904).
Nickel-based alloys (e.g., Inconel, Hastelloy).
Tantalum or titanium for highly aggressive conditions.
These options provide superior resistance but at higher cost.
3.2 Protective Coatings
PTFE (polytetrafluoroethylene) or fluoride-based coatings reduce direct acid–metal contact.
Extend equipment life, particularly for vessels and pipelines.
3.3 Temperature Control
Strictly limit operating temperature in concentrated acid systems.
Thermal design and cooling measures help maintain stainless steel within the passive region.
4. Recommended Comparison Table
Table 1 — Service Suitability of Stainless Steels in Sulfuric Acid
| Material Grade | Dilute H₂SO₄ (<20%) | Medium H₂SO₄ (20–70%) | Concentrated H₂SO₄ (>90%) |
|---|---|---|---|
| 304 SS | Good (low temp) | Not suitable | Limited use at low temp |
| 316 SS | Good (low temp) | Not suitable | Suitable ≤120 °C |
| 317 SS | Better resistance | Partial suitability | Suitable ≤120 °C |
| 310 SS | Better resistance | Partial suitability | Suitable ≤120 °C |
| 904L / Alloys | Excellent | Recommended | Excellent |
5. Practical Industrial Cases (Suggested Additions)
Storage Tanks: 316L tanks safely used for >90% H₂SO₄ at ambient temperature, but failures reported when temperature exceeded 130 °C.
Heat Exchangers: Nickel-based alloys commonly adopted in medium-concentration acid streams.
Piping Systems: PTFE-lined carbon steel pipes often replace stainless steel in the 20–70% “failure zone.”
Conclusion
Stainless steel performance in sulfuric acid is highly dependent on concentration and temperature.
Safe Zones: Dilute (<20%) and concentrated (>90%) acid at low temperature.
Failure Zone: 20–70% concentration range, regardless of steel grade.
Transition Zone: Requires case-by-case evaluation, with 310 and 317 being more reliable.
In practice, engineers must combine correct material selection, temperature control, and protective measures to ensure reliable operation and cost efficiency.
